A kind of synthetic method of 3,4,5-trimethoxybenzoic acid
A technology of trimethoxybenzoic acid and synthesis method, which is applied in chemical instruments and methods, separation/purification of carboxylic acid compounds, preparation of carboxylate salts, etc. , technological production difficulties and other problems, to achieve the effect of reducing waste water production, no ecological environmental risks, and overcoming high risk factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
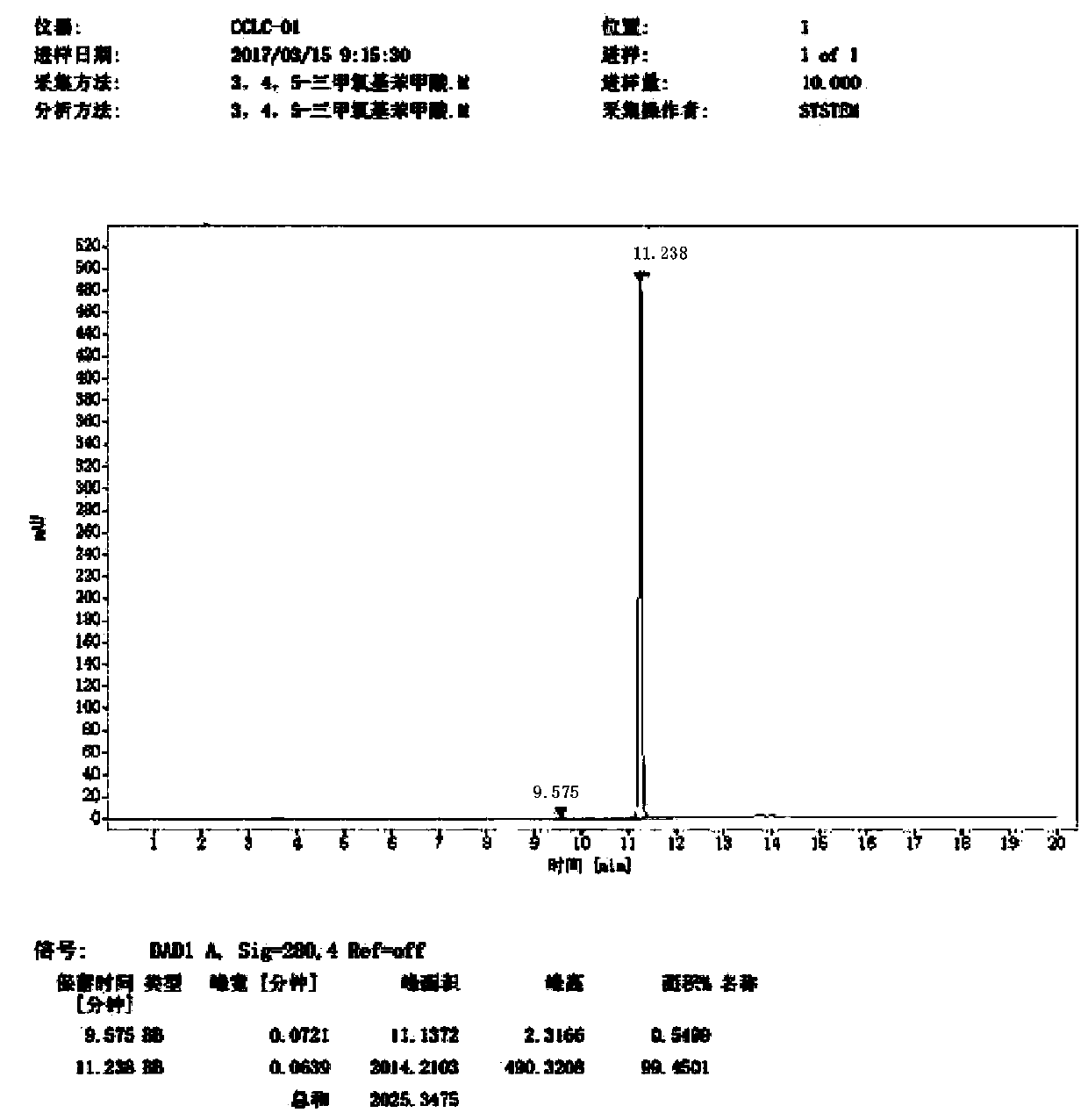
Image
Examples
Embodiment 1
[0025] 1) Add 1313.2 mL (17 mol) of solvent N,N-dimethylformamide, 17 g (0.1 mol) of gallic acid, and catalyst potassium carbonate to the reaction flask protected by inert gas in sequence at 20~25 °C 41.4g (0.3mol) and 103.5g (1.15mol) of dimethyl carbonate, stirred for 0.5~1 hour to fully mix the materials.
[0026] 2) Heat the material in the reaction bottle to 110~120℃, and keep it warm for 8~10 hours.
[0027] 3) After the reaction is completed, initially recover the excess dimethyl carbonate by vacuum distillation at -0.1MPa, 75°C, and then raise the temperature to 90~100°C to distill N,N-dimethylformamide until the bottle forms mushy. The dimethyl carbonate and N,N-dimethylformamide recovered by vacuum distillation are reserved as raw materials in step 1) for recycling.
[0028] 4) Add 576 mL (32mol) of water and 0.84g (0.07mol) of activated carbon into the reaction flask, heat to 80-90°C, keep stirring for 0.5-1 hour, and stop heating.
[0029] 5) Filter the material...
Embodiment 2
[0031] 1) Add 1313.2 mL (17 mol) of solvent N,N-dimethylformamide, 17 g (0.1 mol) of gallic acid, and catalyst potassium carbonate to the reaction flask protected by inert gas in sequence at 20~25 °C 48.3g (0.35mol) and 103.5g (1.15mol) of dimethyl carbonate, stirred for 0.5~1 hour to fully mix the materials.
[0032] 2) Heat the material in the reaction bottle to 110~120℃, and keep it warm for 8~10 hours;
[0033] 3) After the reaction is completed, initially recover the excess dimethyl carbonate by vacuum distillation at -0.1MPa, 75°C, and then raise the temperature to 90~100°C to distill N,N-dimethylformamide until the bottle forms mushy. Dimethyl carbonate and N,N-dimethylformamide recovered by vacuum distillation are reserved as raw materials in step 1) for recycling;
[0034] 4) Add 576 mL (32mol) of water and 0.84g (0.07mol) of activated carbon into the reaction bottle, heat to 80~90℃, keep stirring for 0.5~1 hour, and stop heating;
[0035] 5) Filter the material wh...
Embodiment 3
[0037] 1) Add 1313.2 mL (17 mol) of solvent N,N-dimethylformamide, 17 g (0.1 mol) of gallic acid, and catalyst potassium carbonate to the reaction flask protected by inert gas in sequence at 20~25 °C 55.2g (0.4mol) and 103.5g (1.15mol) of dimethyl carbonate, stirred for 0.5~1 hour to fully mix the materials.
[0038] 2) Heat the material in the reaction bottle to 110~120℃, and keep it warm for 8~10 hours;
[0039] 3) After the reaction is completed, initially recover the excess dimethyl carbonate by vacuum distillation at -0.1MPa, 75°C, and then raise the temperature to 90~100°C to distill N,N-dimethylformamide until the bottle forms mushy. Dimethyl carbonate and N,N-dimethylformamide recovered by vacuum distillation are reserved as raw materials in step 1) for recycling;
[0040] 4) Add 576 mL (32mol) of water and 0.84g (0.07mol) of activated carbon into the reaction bottle, heat to 80~90℃, keep stirring for 0.5~1 hour, and stop heating;
[0041] 5) Filter the material whi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com