Detection method of impurities in lithium hexafluorophosphate
A lithium hexafluorophosphate and detection method technology, applied in the field of chemical analysis, can solve the problems of few qualitative and quantitative reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The present embodiment detects lithium hexafluorophosphate impurity qualitative and quantitative method, comprises the following steps:
[0051] (1) deuterated solvent pretreatment;
[0052] In a nitrogen-filled glove box, the deuterated acetonitrile was dehydrated by molecular sieves to within 10 μg / g (the moisture content was tested by a Karl Fischer moisture meter).
[0053] (2) Calibration
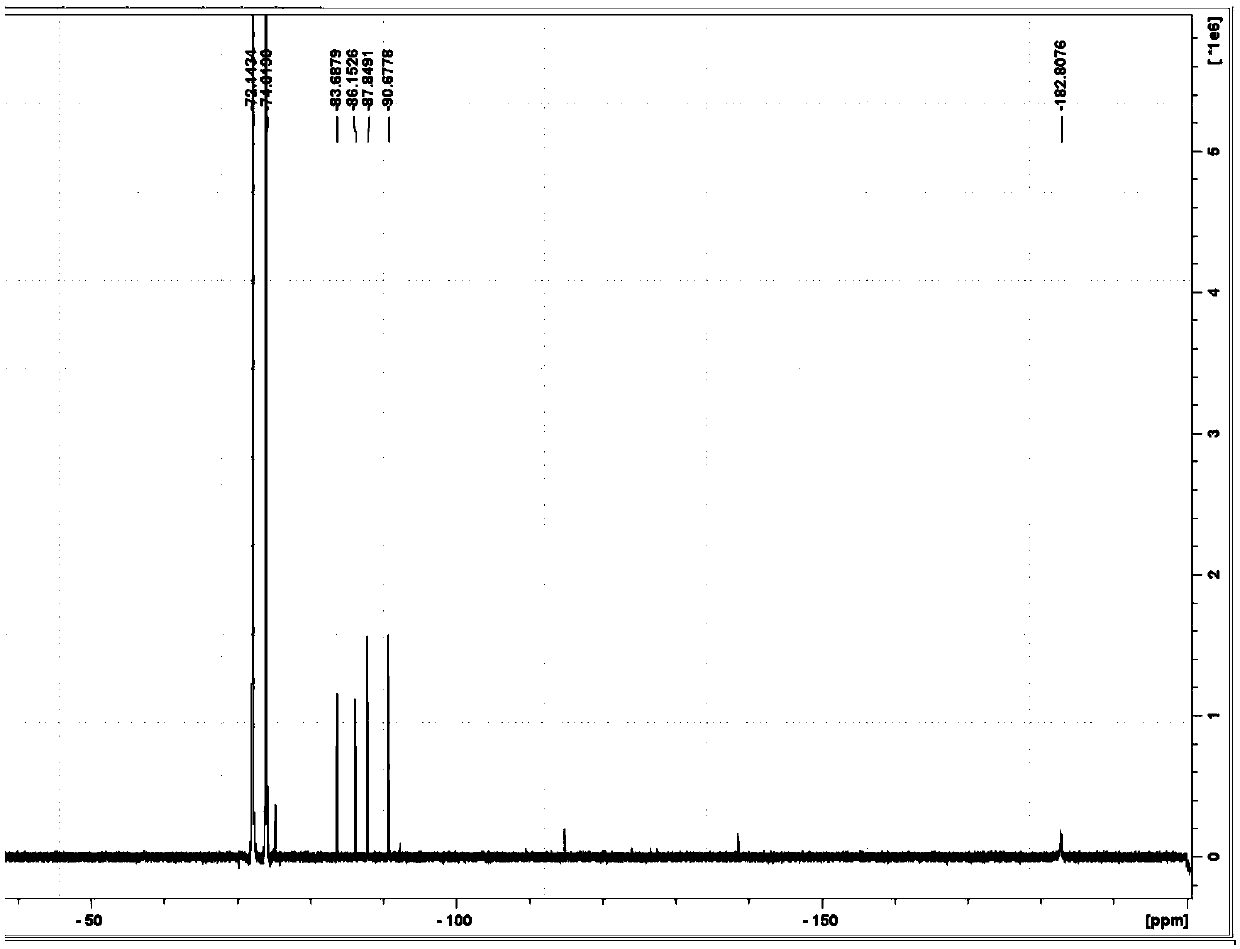
[0054] A coaxial NMR tube is used, and a deuterated acetonitrile solution of hexafluorobenzene and a deuterated acetonitrile solution of lithium hexafluorophosphate with a concentration of 200 μg / g are respectively added to the inner and outer tubes for fluorine spectrum detection; figure 1 shown.
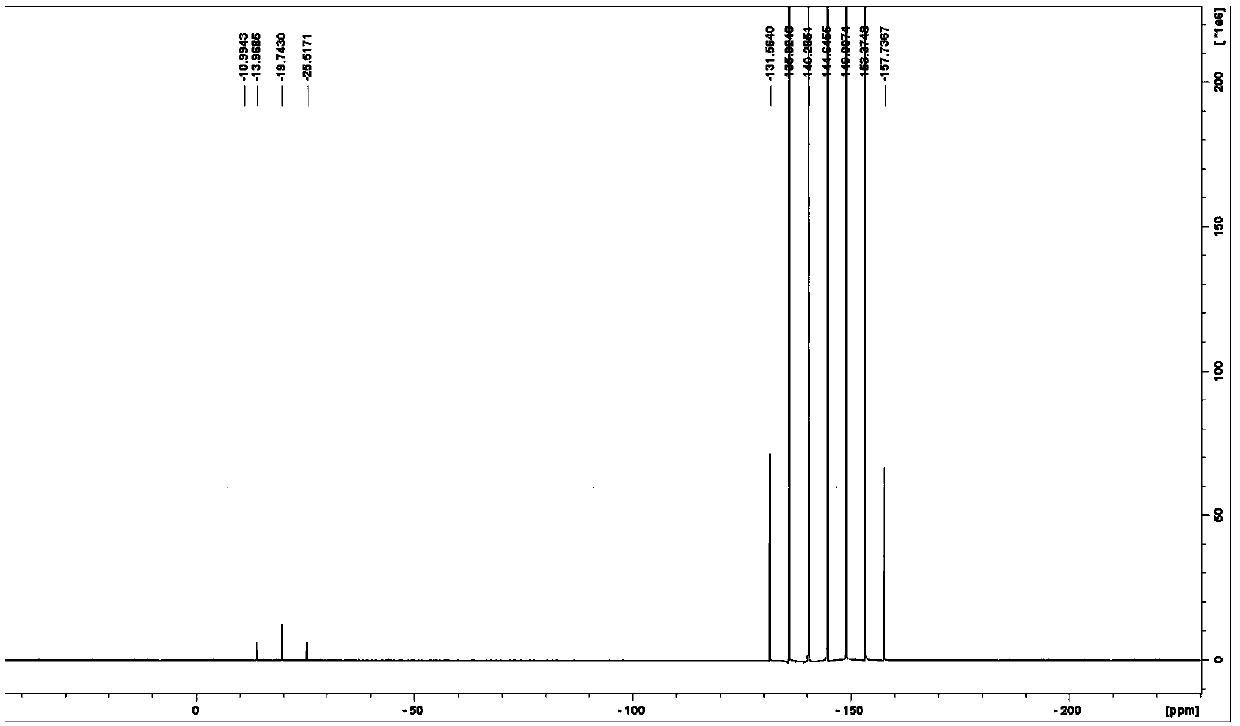
[0055] The deuterated acetonitrile solution of phosphoric acid and the deuterated acetonitrile solution of lithium hexafluorophosphate with a concentration of 1000 μg / g were respectively added into the inner and outer tubes for phosphorus spectrum detection; figure 2 shown.
[0...
Embodiment 2
[0079] The present embodiment detects lithium hexafluorophosphate impurity qualitative and quantitative method, comprises the following steps:
[0080] (1) deuterated solvent pretreatment;
[0081] In a nitrogen-filled glove box, the deuterated acetone was refluxed with calcium hydride to remove water to within 10 μg / g (the moisture content was tested by a Karl Fischer moisture meter).
[0082] (2) Calibration
[0083] A coaxial nuclear magnetic tube is used, and a deuterated acetone solution of hexafluorobenzene and a deuterated acetone solution of lithium hexafluorophosphate with a concentration of 200 μg / g are respectively added to the inner and outer tubes for fluorine spectrum detection;
[0084] The deuterated acetone solution of phosphoric acid and the deuterated acetone solution of lithium hexafluorophosphate with a concentration of 1000 μg / g were respectively added to the inner and outer tubes for phosphorus spectrum detection;
[0085] (3) Lithium hexafluorophospha...
Embodiment 3
[0089] The present embodiment detects lithium hexafluorophosphate impurity qualitative and quantitative method, comprises the following steps:
[0090] (1) deuterated solvent pretreatment;
[0091] In a nitrogen-filled glove box, the deuterated dimethyl sulfoxide was dehydrated by molecular sieves to within 10 μg / g (the moisture content was tested by a Karl Fischer moisture meter).
[0092] (2) Calibration
[0093] A coaxial NMR tube is used, and a deuterated dimethyl sulfoxide solution of hexafluorobenzene and a deuterated dimethyl sulfoxide solution of lithium hexafluorophosphate are added to the inner and outer tubes with a concentration of 200 μg / g, respectively, for fluorine spectrum detection;
[0094] A coaxial nuclear magnetic tube is used, and a deuterated dimethyl sulfoxide solution of phosphoric acid and a deuterated dimethyl sulfoxide solution of lithium hexafluorophosphate are added to the inner and outer tubes with a concentration of 1000 μg / g, respectively, for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com