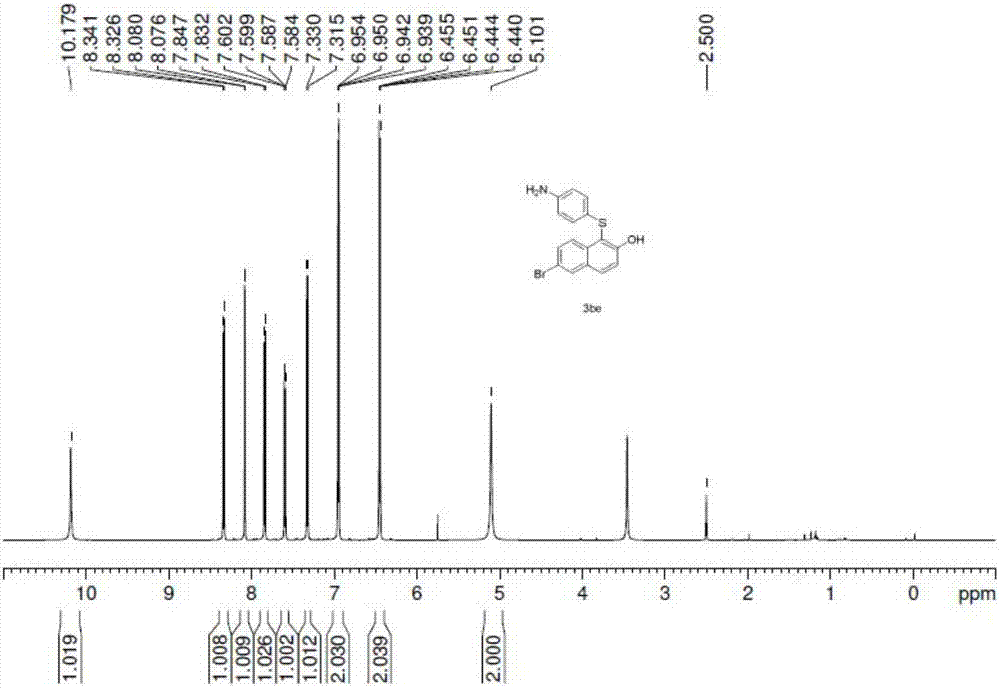
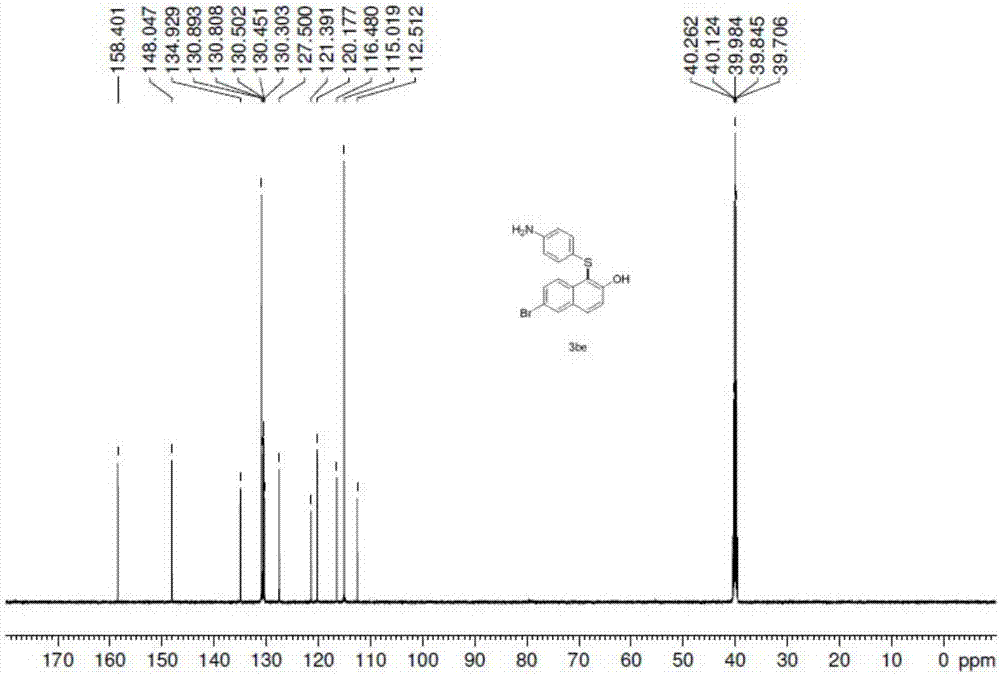
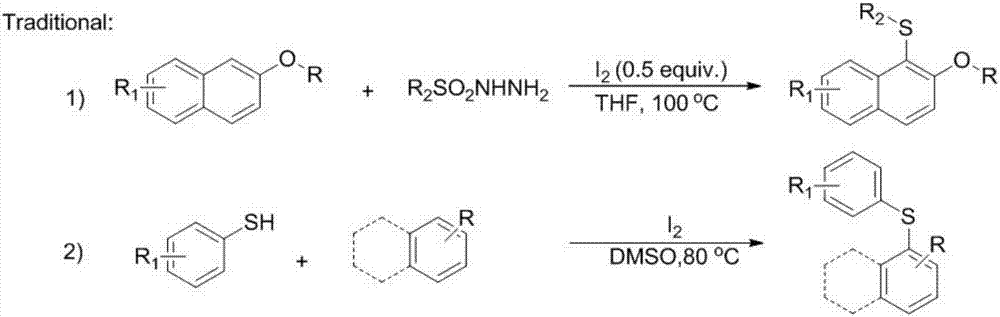
Synthetic method for aryl thioether compounds
A technology of thiol compounds and aryl sulfides, which is applied in the field of synthesis of oxygen and nitrogen compounds, can solve the problems of high cost, reduce waste liquid, be easy to operate, and improve efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation method of aryl sulfide compound is as follows:
[0029]
[0030] Add β-naphthol compound (0.5mmol), p-cresylthiol (1.5mmol), sulfonated cobalt phthalocyanine (0.025mmol) in the dry 25mL Schlenk reaction tube, add water 2mL, flush into oxygen, heat to reflux, After reacting for 18 hours, after the reaction, extract with ethyl acetate, dry the extract with anhydrous sodium sulfate, concentrate under reduced pressure to remove the solvent, and separate by column chromatography to obtain the target product.
Embodiment 2
[0032] The preparation method of aryl sulfide compound is as follows:
[0033]
[0034] Add β-naphthol compound (0.5mmol), o-bromothiophenol (83.5mg, 1.5mmol) and copper tetracarboxyphthalocyanine (0.025mmol) to the dry 25mL Schlenk reaction tube, add water 2mL, flush into oxygen, Heat to reflux and react for 24 hours. After the reaction, extract with ethyl acetate, dry the extract with anhydrous sodium sulfate, concentrate under reduced pressure to remove the solvent, and separate by column chromatography to obtain the target product.
Embodiment 3
[0036] The preparation method of aryl sulfide compound is as follows:
[0037]
[0038] Add 6-bromo-2-naphthol compound (0.5mmol), hexanethiol (1.5mmol), sulfonated iron phthalocyanine (0.025mmol) into a dry 25mL Schlenk reaction tube, add 2mL of water, flush into oxygen, and heat Return to reflux and react for 24 hours. After the reaction, extract with ethyl acetate, dry the extract with anhydrous sodium sulfate, concentrate under reduced pressure to remove the solvent, and separate by column chromatography to obtain the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com