Detection kit, primer and probe for simultaneously detecting and identifying classical swine fever, African swine fever and swine vesicular disease
A technology for classical swine fever virus and African swine fever virus, which is applied in the field of inspection and quarantine, can solve the problems of large detection workload, low sensitivity, and difficult to determine results, achieve easy automation, good coverage and versatility, and save detection time. and cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Composition and use of embodiment 1 test kit
[0039] 1. The composition of the kit
[0040] Table 2 Composition of the kit
[0041]
[0042]
[0043] Among them, 2×Multiplex RT-PCR buffer and Multiplex enzymes were purchased from Path-ID TMMultiplex one-step RT-PCR kit from AB Company.
[0044] The primer mix includes 3 forward primers and 2 reverse primers for CSFV, 2 forward primers and 3 reverse primers for ASFV, and 1 forward primer and 1 reverse primer for SVDV. The primer sequences are shown in Table 1 , the working concentration of the mixed primers is 400nM, and the primers are synthesized by Dalian Bao Biological Company.
[0045] The CSFV probe mixture includes 2 probes for CSFV, 1 probe for ASFV and 1 probe for SVDV. The sequences of the probes are shown in Table 1, and the concentrations are 200nM. The probes were synthesized by Dalian Bao Biological Company.
[0046] The negative control is sterile water without nucleic acid.
[0047] The CSFV po...
Embodiment 2
[0092] Embodiment 2, the sensitivity test of kit
[0093] 1. Materials
[0094] Classical swine fever virus is preserved by our laboratory, and African swine fever virus and porcine vesicular disease virus are recombinant plasmids in vitro.
[0095] 2. Method
[0096] 1) Preparation of positive standard
[0097] Method as described in Example 1.
[0098] 2) Quantitative determination of standard
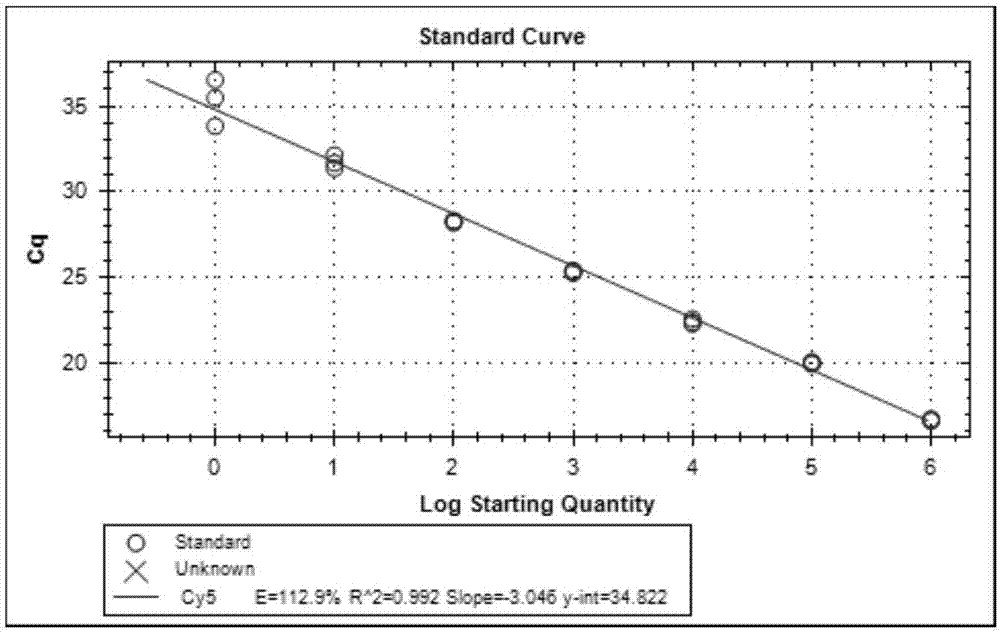
[0099] Take the prepared in vitro transcribed cRNA and in vitro recombinant DNA and make 200-fold dilutions with RNase-free sterilized water, measure their absorbance values at 260nm and 280nm (OD260 and OD280) with an ultraviolet spectrometer, and calculate the concentration of the sample to be tested and purity. Pure DNA: OD260 / OD280≈1.8 (>1.9, indicating RNA contamination; 2.0, indicating that there may be residual isothiocyanate); pure RNA: 1.72.0 indicates that there may be residual isothiocyanate). Concentration of DNA sample (μg / μL): OD260×dilution factor×50 / 1000; conc...
Embodiment 3
[0115] Embodiment 3, the specificity test of kit
[0116] 1 material
[0117] The viruses used in this experiment are listed in Table 6.
[0118] Table 6 Viruses and nucleic acids used in the specificity test research process
[0119] Virus source Classical swine fever virus (CSFV) The lab saves African swine fever virus (ASFV) USDA Exotic Disease Center Porcine vesicular disease virus recombinant plasmid in vitro Prepared in this laboratory Porcine circovirus type 2 (PCV-2) The lab saves Pseudorabies virus (PRV) The lab saves Porcine parvovirus (PPV) The lab saves transmissible gastroenteritis virus (TGEV) The lab saves
[0120] 2. Method
[0121] 2.1 Use the primers and probes of classical swine fever virus, African swine fever virus and porcine vesicular disease virus to perform fluorescent RT-PCR detection on the other 6 viral nucleic acids in the table to verify the specificity of the primers and probes. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com