Camptothecin prodrug, and preparation method and application thereof
A kind of technology of camptothecin and prodrug, applied in the field of small molecule amphiphilic drugs and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
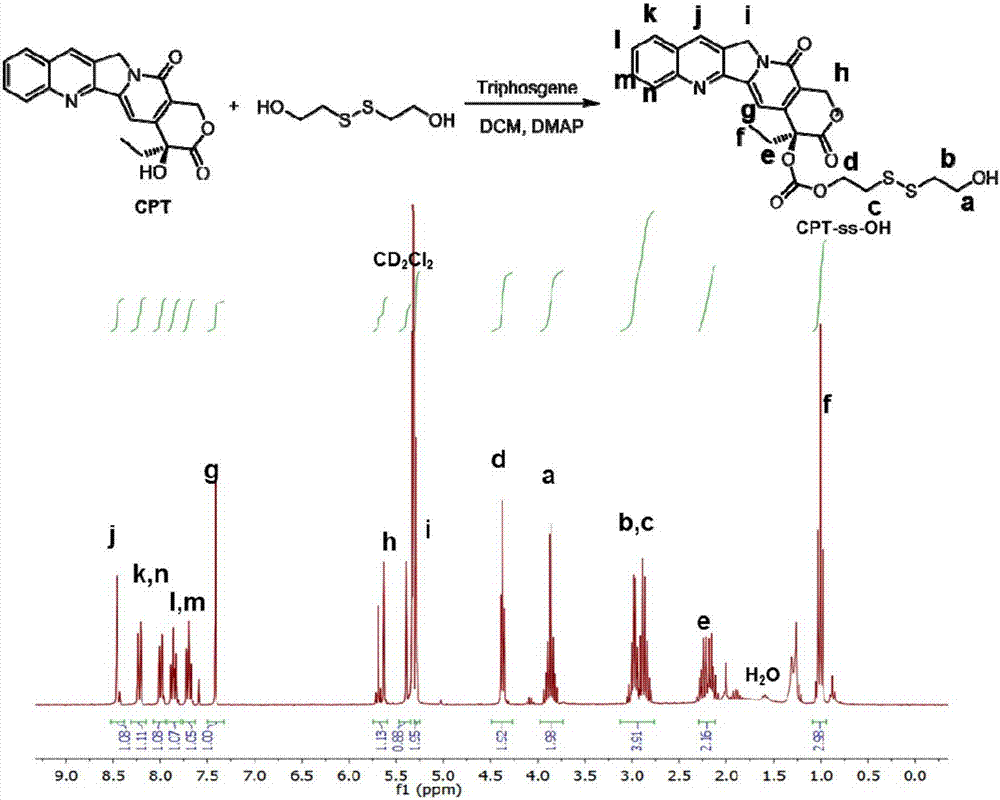
[0093] Embodiment 1: the preparation of CPT-ss-OH
[0094] Under stirring, a solution of 4-dimethylaminopyridine (1.05 g, 8.60 mmol) in 10 mL of dichloromethane was added dropwise to a solution of camptothecin (1.0 g, 2.87 mmol) and triphosgene (0.315 g, 1.06 mmol). The mixture was suspended in anhydrous dichloromethane (200 mL). After stirring for 30 minutes, 2,2'-dithiodiethanol (8.60 g, 55.8 mmol) in anhydrous tetrahydrofuran (25 mL) was added and the reaction mixture was stirred at room temperature overnight. The mixture was washed with 50 mM aqueous hydrochloric acid (2×100 mL), water (1×100 mL) and saturated brine (1×100 mL). The organic layer was separated and dried over anhydrous sodium sulfate. The solution was concentrated by rotary evaporator and purified by flash chromatography using a prepacked silica column. Yield: 1.05 g (69% yield). 1 H NMR (300MHz, CD 2 Cl 2 )δ8.46(s, 1H), 8.22(d, J=8.3Hz, 1H), 7.99(dd, J=8.2, 1.1Hz, 1H), 7.86(ddd, J=6.9, 6.5Hz, 1H), 7....
Embodiment 2
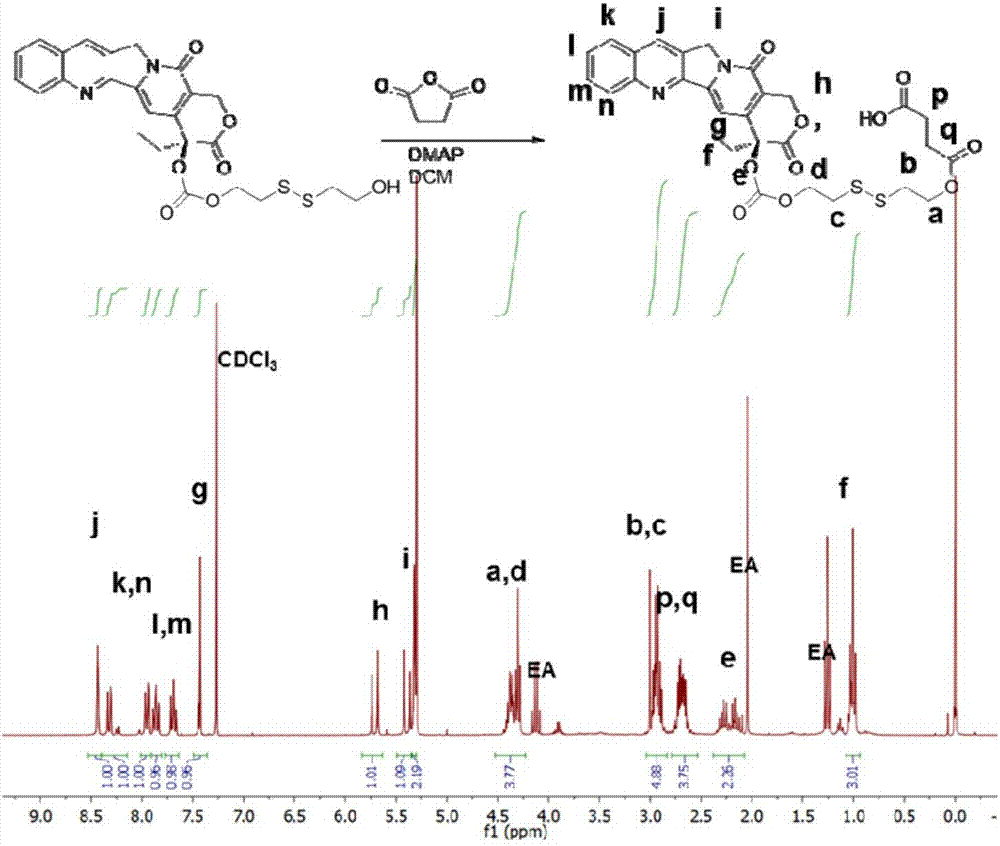
[0095] Embodiment 2: Preparation of CPT-ss-COOH
[0096] Succinic anhydride (400 mg, 4.00 mmol), CPT-ss-OH (204 mg, 0.386 mmol) prepared in Example 1 and 4-dimethylaminopyridine (19.6 mg, 0.161 mmol) were dissolved in anhydrous dichloromethane under stirring (200mL). The reaction mixture was stirred at room temperature overnight, then washed with water (1×100 mL), 50 mM aqueous hydrochloric acid (1×100 mL) and saturated brine (1×100 mL). The organic layer was separated and dried over anhydrous sodium sulfate. The solution was concentrated by rotary evaporator and purified by flash chromatography using a prepacked silica column. Yield: 201 mg (83% yield). 1 H NMR (300MHz, CDCl 3 )δ8.44(s, 1H), 8.32(d, J=8.4Hz, 1H), 7.95(d, J=8.2Hz, 1H) (ddd, J=8.5, 6.9, 1.4Hz, 1H), 7.69( ddd, J=8.1, 7.0, 1.1Hz, 1H), 7.43(s, 1H), 5.71(d, J=17.3Hz, 1H), 5.39(d, J=17.3Hz, 1H), 5.32(s, 2H) , 4.46-4.25 (m, 4H), 3.00-2.86 (m, 4H), 2.79-2.61 m, 2H), 2.18 (m, J=2H), 1.01 (t, J=7.5, 3H). ESI-MS m...
Embodiment 3
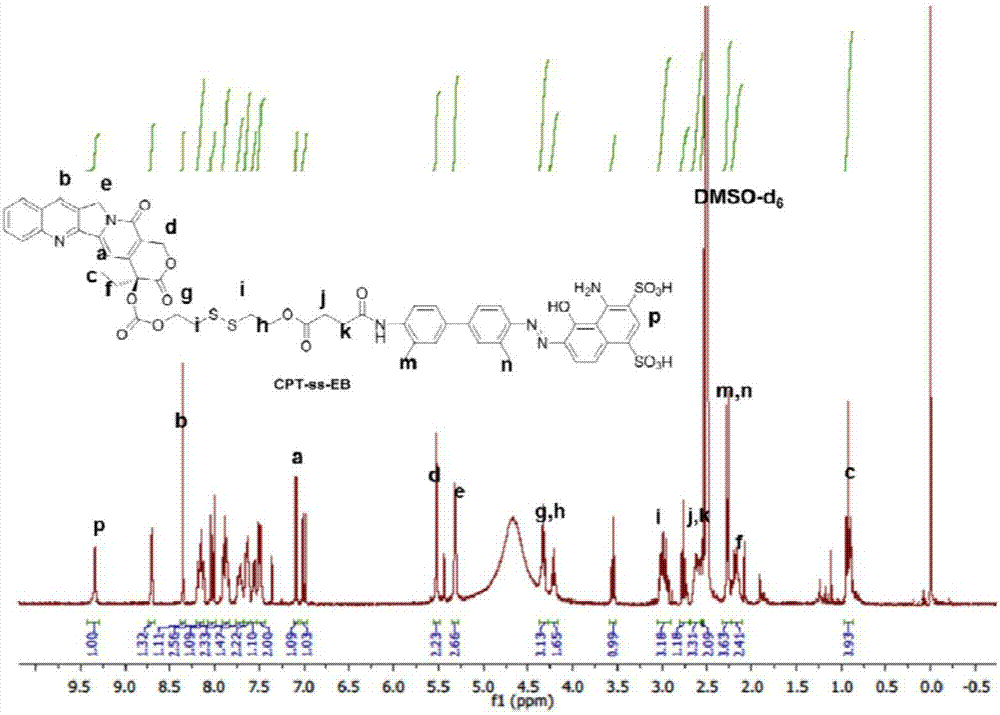
[0097] Embodiment 3: Preparation of CPT-ss-EB
[0098] CPT-ss-COOH (58mg, 0.092mmol), EB-amine (25mg, 0.046mmol) prepared in Example 2, benzotriazol-1-yl-oxytripyrrolidinylphosphonium hexafluorophosphate (PyBOP, 48mg , 0.092mmol) and N,N-diisopropylethylamine (DIPEA, 59mg, 0.46mmol) were mixed in dimethylformamide and stirred under nitrogen for 2 days. The reaction was quenched by adding excess acetic acid and purified by preparative high performance liquid phase using acetonitrile and 0.2% acetic acid in water (gradient: 5-95% acetonitrile). The collected purified product was lyophilized and stored at -20°C for later use. Yield: 21 mg (40% yield). ESI-MS m / z: Calculated 1152, found 1151 (M-H). 1 H NMR (300MHz, DMSO) δ9.34(s, 1H), 8.70(d, J=3.7Hz, 1H), 8.35(s, 1H), 8.21-8.10(m, 3H), 8.02(d, 9.9Hz , 1H), 7.88 (dt, J=8.4, 4.1Hz, 2H), 7.73 (dd, J=6.9, 4.1Hz, 1H), 7.64 (dd, J=4.0Hz, J=6.5Hz, 1H), 7.50 (d, J=5.2Hz, 2H), 7.09(d, J=2.2Hz, 1H), 7.00(d, J=10Hz, 1H), 5.56-5.32(m, 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com