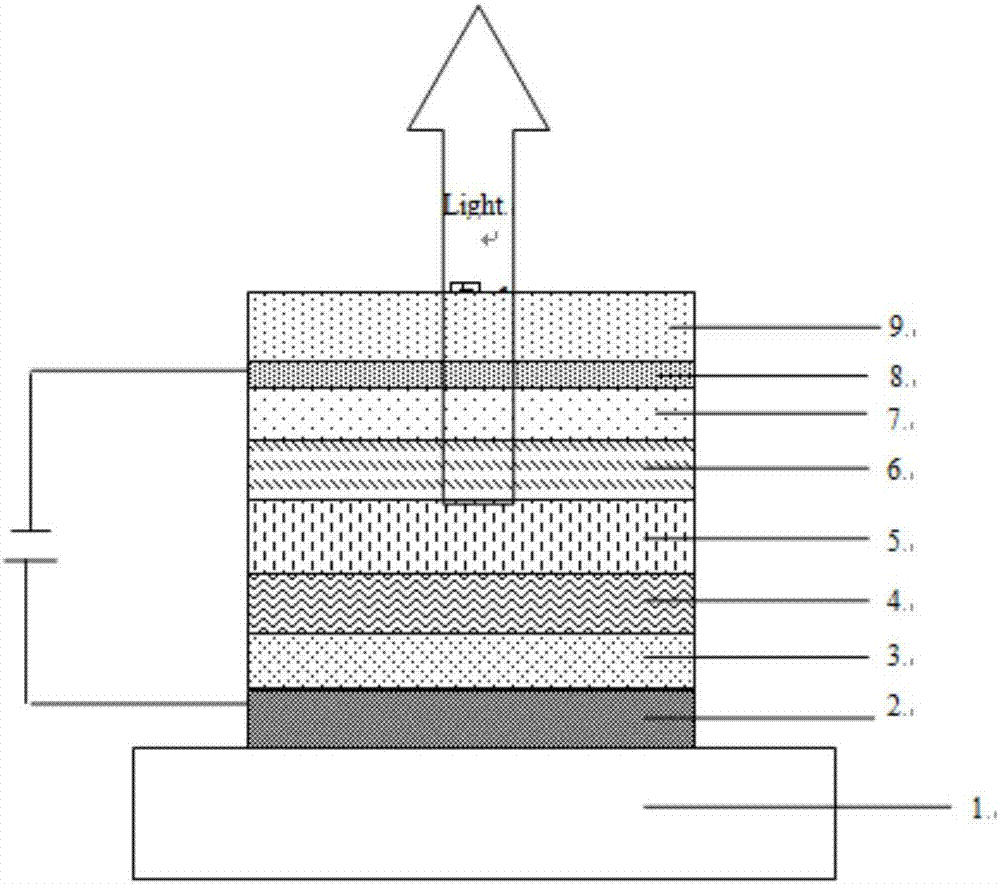
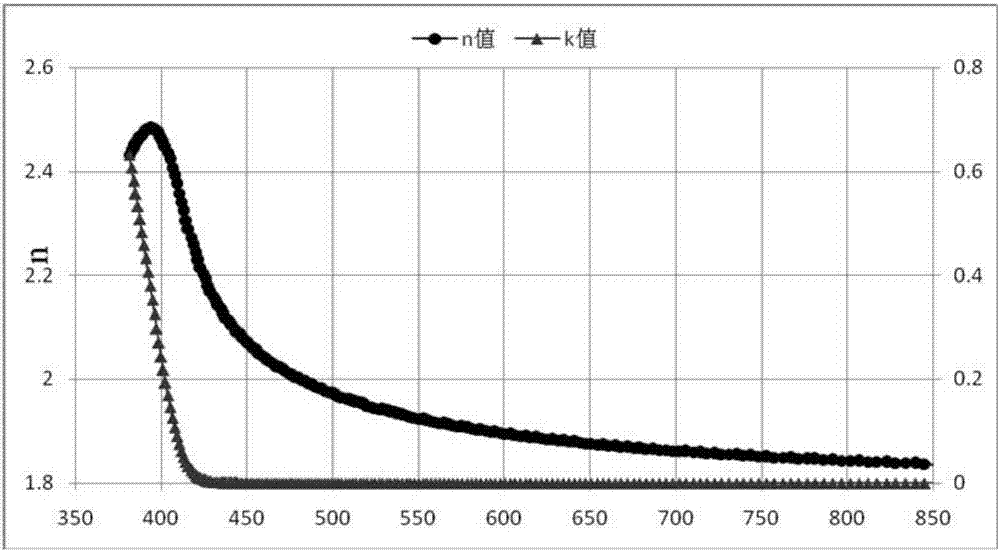
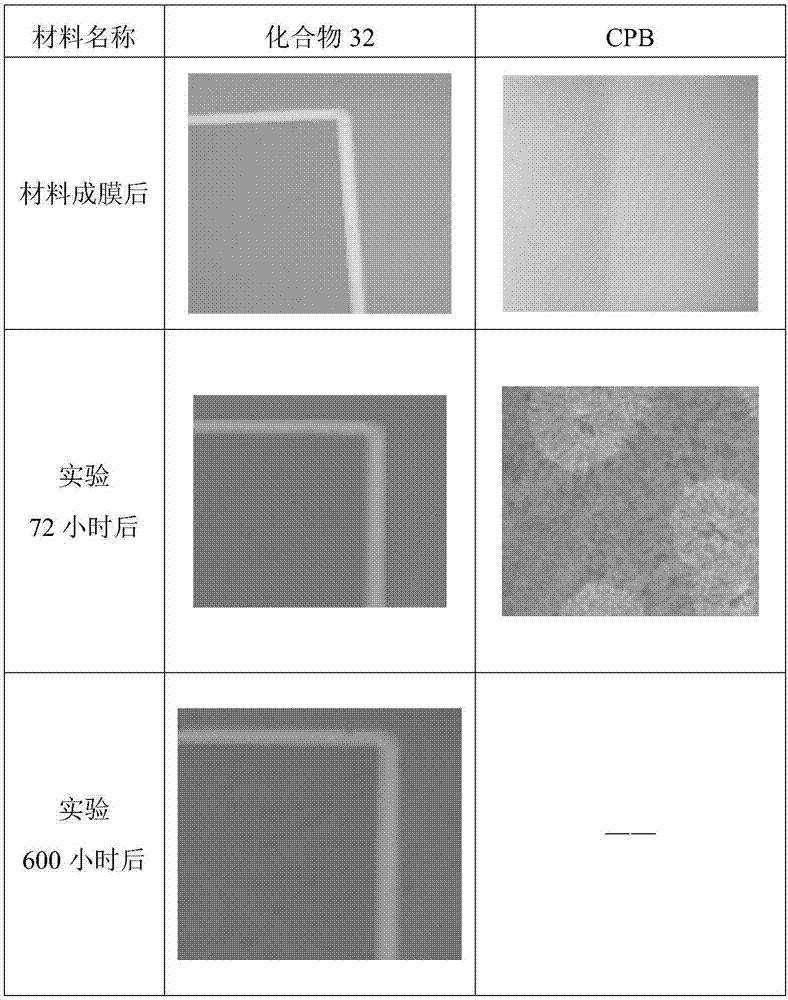
Organic compound taking triazine and benzimidazole as cores and application thereof to organic electroluminescent device
An organic compound and benzimidazole technology, applied to organic electroluminescent devices, in the field of organic compounds, can solve the problems of complex production process, affecting the angular distribution of OLED radiation spectrum, etc., and achieve high electron mobility and good application effect. and industrialization prospects, the effect of high refractive index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: the synthesis of intermediate I
[0048]
[0049] Under nitrogen atmosphere, the raw material 2,4,6-trichloro-1,3,5-triazine was weighed and dissolved in tetrahydrofuran, and Ar 1 The boric acid compound and tetrakis (triphenylphosphine) palladium are added, the mixture is stirred, and then saturated potassium carbonate aqueous solution is added, and the mixed solution of the above reactants is heated and refluxed at a reaction temperature of 70-90° C. for 10-20 hours; after the reaction is completed, After cooling, the mixture was extracted with dichloromethane, the extract was dried over anhydrous sodium sulfate, and concentrated under reduced pressure, and the concentrated solid was purified by a silica gel column to obtain compound intermediate I;
[0050] The 2,4,6-trichloro-1,3,5-triazine and Ar 1 -B(OH) 2 The molar ratio of Pd(PPh 3 ) 4 The molar ratio to 2,4,6-trichloro-1,3,5-triazine is 0.005~0.05:1, K 2 CO 3 The molar ratio of 2,4,6-tric...
Embodiment 2
[0059] Embodiment 2: intermediate Synthesis
[0060] When Ar 2 or Ar 3 When expressed as a structure of general formula (2),
[0061]
[0062] (1) In a 250mL three-necked flask, feed nitrogen, add 0.02mol raw material 2-bromo-benzimidazole, 0.03mol iodobenzene, 0.04mol sodium hydride, 0.004mol cuprous iodide and 0.01mol o-phenanthroline and dissolve in In 100ml 1,3-dimethyl-2-imidazolidinone, stir the reaction for 20-30h, after the reaction, add water and extract with dichloromethane, dry the organic layer with anhydrous sodium sulfate, and use petroleum ether and ethyl acetate The mixture was rinsed with eluent, the volume ratio of petroleum ether and ethyl acetate in the eluent was 1:100, and purified by column chromatography to obtain intermediate M;
[0063]
[0064] (2) Under a nitrogen atmosphere, weigh the intermediate M and dissolve it in tetrahydrofuran, and then dissolve the Br-Ar 2 -B(OH) 2 And tetrakis (triphenylphosphine) palladium is added, the mixtu...
Embodiment 3
[0098] Embodiment 3: the synthesis of compound 1:
[0099]
[0100] In a 250mL three-necked flask, blow nitrogen gas, add 0.01mol of intermediate A1, 150ml of DMF, 0.03mol of intermediate B1, 0.0002mol of palladium acetate, stir, and then add 0.02mol of K 3 PO 4 The aqueous solution was heated to 150°C, refluxed for 24 hours, sampled and plated, and the reaction was complete. Cool naturally, extract with 200ml of dichloromethane, separate layers, dry the extract with anhydrous sodium sulfate, filter, rotate the filtrate, and purify through a silica gel column to obtain the target product with a HPLC purity of 99.1% and a yield of 72.1%.
[0101] Elemental analysis structure (molecular formula C 51 h 33 N 7 ): theoretical value C, 82.35; H, 4.47; N, 13.18; test value: C, 82.37; H, 4.48; N, 13.15. ESI-MS(m / z)(M + ): The theoretical value is 743.28, and the measured value is 743.62.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com