Method for synthesizing hydroxylamine hydrochloride from nitromethane and hydrochloric acid through phase transfer
A technology of nitromethane and hydroxylamine hydrochloride, applied in chemical instruments and methods, hydroxylamine, nitrogen and non-metallic compounds, etc., can solve the problems of low conversion rate and long reaction time, and achieve low production cost, fast reaction speed, and yield high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
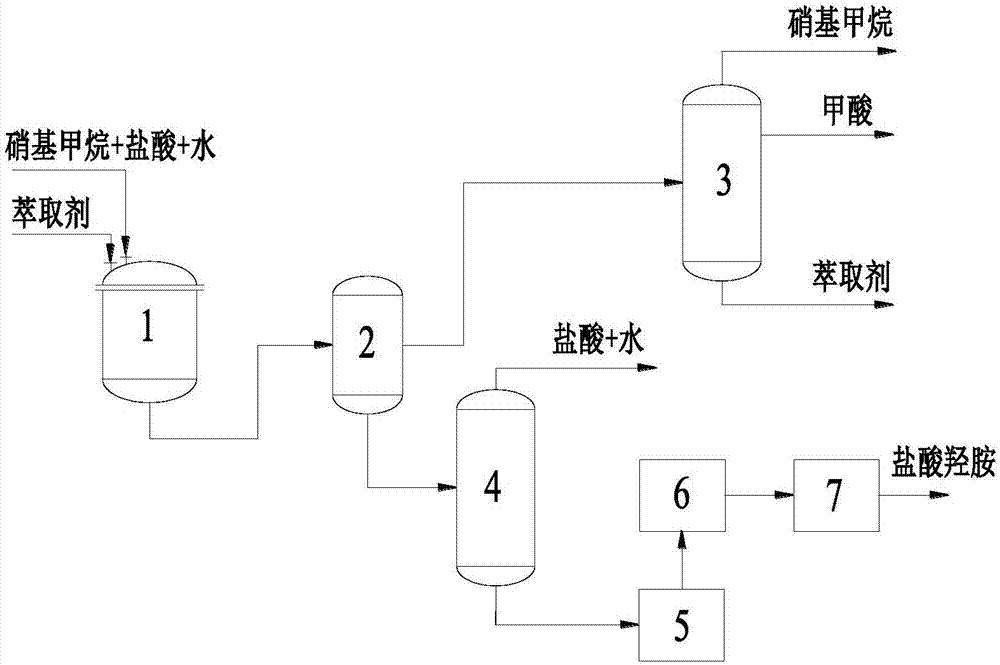
[0034] like figure 1 As shown, nitromethane and hydrochloric acid are added in the synthesis reactor 1 in a molar ratio of 1:1.5, stirred and mixed, and the extractant n-octanol is added, and the molar ratio of nitromethane and n-octanol is 1:1, and the temperature is raised to 130 After reacting at ℃ for 36 hours, the heating is stopped, and the reaction product is sent to the phase separator 2. After phase separation, the oil phase enters the rectification tower 3. After rectification, formic acid is a by-product, and nitromethane and octanol are recovered and recycled. The aqueous phase coming out of the phase separator 2 enters the concentration tower 4, and the concentration adopts vacuum distillation, and the distillation pressure is - 0.1MPa (G), concentrated distilled water and hydrochloric acid are recycled, the concentrated solution enters the crystallizer 5 to crystallize, then enters the filter 6 to filter out the solid, washes with anhydrous ether for 3 times, and...
Embodiment 2
[0040] like figure 1 As shown, nitromethane and hydrochloric acid are added in the synthesis reactor 1 in a molar ratio of 1:3, stirred and mixed, and the extractant n-hexanol is added, and the molar ratio of nitromethane and n-hexanol is 1:1, and the temperature is raised to 130 ℃ reaction 36h. In addition, the same method as in Example 1 was used to obtain hydroxylamine hydrochloride crystals with a yield of 76.22%.
Embodiment 3
[0046] like figure 1 As shown, nitromethane and hydrochloric acid are added in the synthetic reactor 1 in molar ratio 1:2, stir and mix, add extraction agent trioctylamine, the mol ratio of nitromethane and trioctylamine is 1:1, heat up to The temperature was 130°C for 36h. In addition, the same method as in Example 1 was used to obtain hydroxylamine hydrochloride crystals with a yield of 74.12%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com