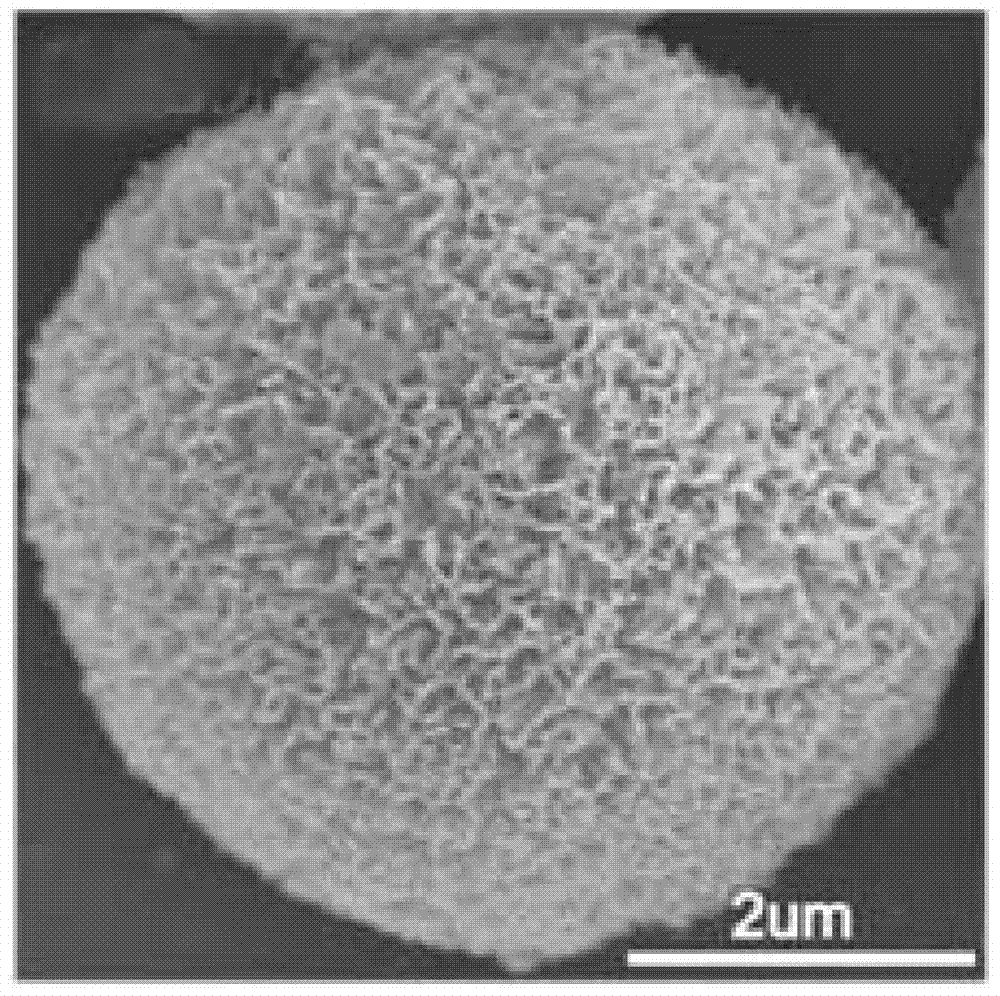
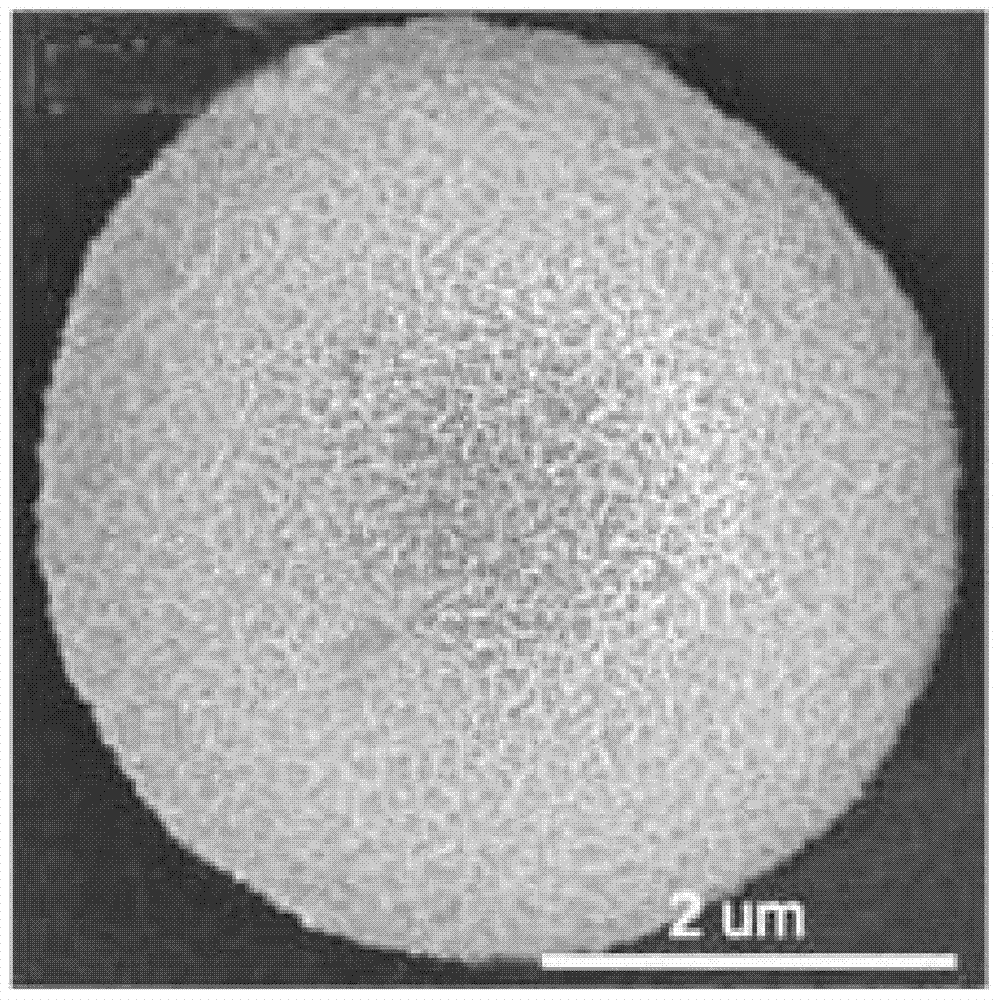
Graphite phase carbon nitride porous microsphere and preparation method thereof
A technology of graphite phase carbon nitride and porous microspheres, applied in the field of photocatalytic materials, can solve the problems of serious recombination of photogenerated electron-hole pairs, low utilization rate of visible light, and limited popularization and application, and achieve regular structure, low cost, Effects from a wide range of sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) At room temperature, put 5g of cyanamide in a crucible with a lid, heat it to 450°C in a horse-boiling furnace under air atmosphere, and roast it for 6h at a heating rate of 2°C / min. After cooling, a yellow solid powder graphite phase nitrogen is obtained. carbonization;
[0037](2) Disperse 0.5 g of the graphite phase carbon nitride obtained in 10 mL of sulfuric acid with a mass concentration of 40%, stir at 110° C. for 0.4 h, and cool down to room temperature after the graphite phase carbon nitride is completely dissolved to obtain graphite phase nitrogen carbonized sulfuric acid solution;
[0038] (3) Dilute the sulfuric acid solution of the obtained graphitic carbon nitride to 1 mg / mL with deionized water, then lower the temperature to 0° C., and let it stand for 5 hours to obtain a white precipitate;
[0039] (4) The obtained white precipitate was centrifuged, washed with deionized water until neutral, and dried at 80° C. for 24 hours to obtain graphite-phase ...
Embodiment 2
[0042] (1) At room temperature, put 5g of dicyandiamide in a crucible with a lid, heat it to 550°C in a horse-boiler furnace under air atmosphere, roast for 3h, the heating rate is 5°C / min, and obtain a yellow solid powder graphite phase after cooling carbon nitride;
[0043] (2) Disperse 1 g of the graphite phase carbon nitride obtained in 10 mL of sulfuric acid with a mass concentration of 60%, stir at 100° C. for 1 h, and cool down to room temperature after the graphite phase carbon nitride is completely dissolved to obtain the graphite phase carbon nitride sulfuric acid solution;
[0044] (3) Dilute the obtained sulfuric acid solution of graphitic carbon nitride to 1.5 mg / mL with deionized water, then lower the temperature to 10° C., and let stand for 5 hours to obtain a white precipitate;
[0045] (4) The obtained white precipitate was centrifuged, washed with deionized water until neutral, and dried at 80° C. for 24 hours to obtain graphite-phase carbon nitride porous m...
Embodiment 3
[0048] (1) At room temperature, put 5g of urea in a crucible with a lid, heat it to 650°C in a horse-boiling furnace under air atmosphere, and bake it for 1h at a heating rate of 20°C / min. After cooling, a yellow solid powder graphite phase nitriding is obtained. carbon;
[0049] (2) Disperse 1.5 g of the graphite phase carbon nitride obtained in 10 mL of sulfuric acid with a mass concentration of 80%, stir at 100° C. for 2 hours, and cool down to room temperature after the graphite phase carbon nitride is completely dissolved to obtain graphite phase nitride sulfuric acid solution of carbon;
[0050] (3) Dilute the obtained sulfuric acid solution of graphitic carbon nitride to 2.2 mg / mL with deionized water, then lower the temperature to 20° C., and let stand for 20 hours to obtain a white precipitate;
[0051] (4) The obtained white precipitate was centrifuged, washed with deionized water until neutral, and dried at 80° C. for 24 hours to obtain graphite-phase carbon nitrid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com