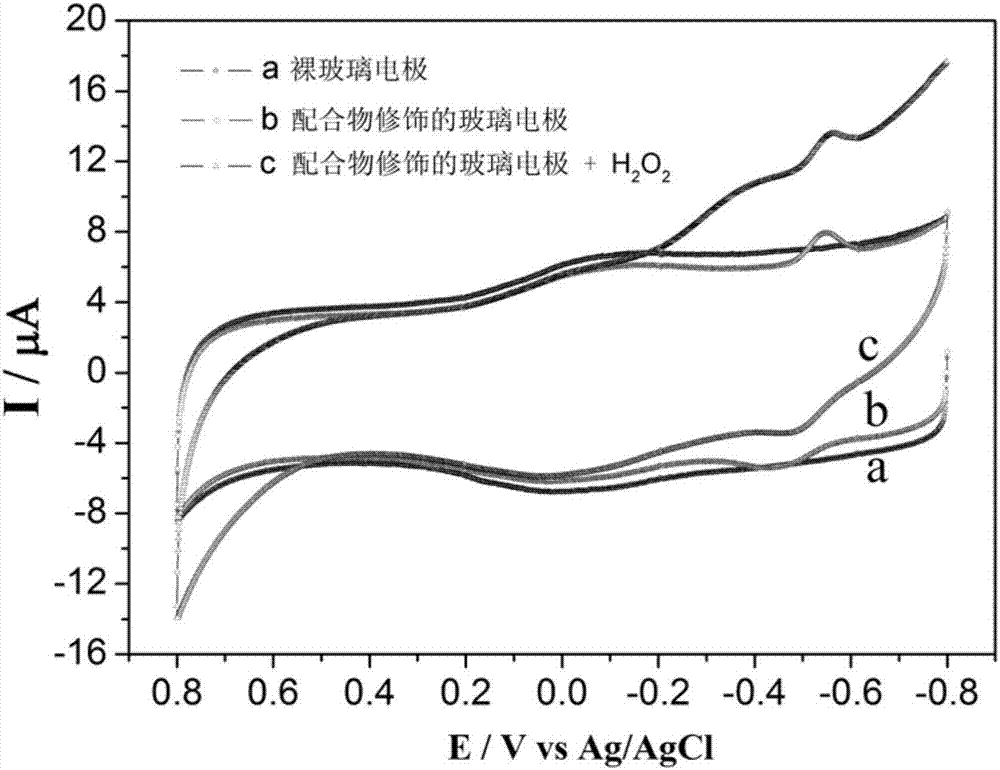
Cobalt complex with electrocatalytic activity on formaldehyde and preparation method thereof
A technology of cobalt complexes and electrocatalysis, applied in the field of material chemistry, can solve problems such as poor safety, high energy consumption, complex operation and analysis, etc., achieve accurate spatial structure, facilitate electronic transition and energy transfer, and have good electrocatalytic activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Weigh CoCl 2 ·6H 2 O (0.3mmol, 0.0714g), 5-nitroorotate potassium monohydrate (0.3mmol, 0.0772g) is placed in a beaker, add water 10mL, heat, stir to make it dissolve, obtain the reaction mixture aqueous solution; The reaction mixture aqueous solution Transfer to a 25mL stainless steel reaction kettle with polytetrafluoroethylene lining, seal the reaction kettle and place it in a blast drying oven, heat and react at 100°C for 72 hours, cool to room temperature after the reaction is completed, open the reaction kettle to obtain brown-red block crystals ; Take out the brownish-red blocky crystal and dry it naturally to obtain the cobalt complex.
Embodiment 2
[0020] Weigh CoCl 2 ·6H 2 O (0.3mmol, 0.0714g), 5-nitroorotate potassium monohydrate (0.6mmol, 0.154g) is placed in the beaker, add water 15mL, heat, stir to make it dissolve, obtain the reaction mixture aqueous solution; The reaction mixture aqueous solution Transfer to a 25mL stainless steel reaction kettle with polytetrafluoroethylene lining, seal the reaction kettle and place it in a blast drying oven, heat and react at 120°C for 24 hours, cool to room temperature after the reaction, open the reaction kettle to obtain brown-red block crystals ; Take out the brownish-red blocky crystal and dry it naturally to obtain the cobalt complex.
Embodiment 3
[0022] Weigh CoCl 2 ·6H 2 O (0.2mmol, 0.0475g), 5-nitroorotic potassium monohydrate (0.3mmol, 0.0772g) is placed in a beaker, add water 13mL, heat, stir to make it dissolve, obtain the reaction mixture aqueous solution; The reaction mixture aqueous solution Transfer to a 25mL stainless steel reaction kettle with polytetrafluoroethylene lining, seal the reaction kettle and place it in a blast drying oven, heat and react at 110°C for 48 hours, cool to room temperature after the reaction, open the reaction kettle to get brown-red block crystals ; Take out the brownish-red blocky crystal and dry it naturally to obtain the cobalt complex.
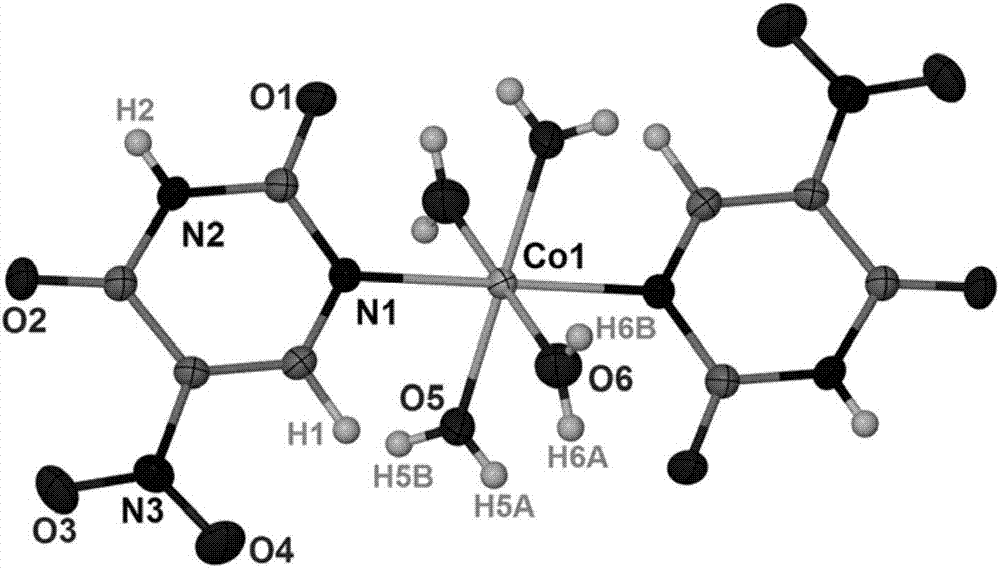
[0023] The cobalt complexes prepared in Examples 1-3 were subjected to structural testing, and the results showed that the carboxyl group of 5-nitroorotic acid was removed during the reaction, and its nitrogen was used to coordinate with cobalt ions to form a new cobalt coordination compound. Its chemical formula of the cobalt complex that th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com