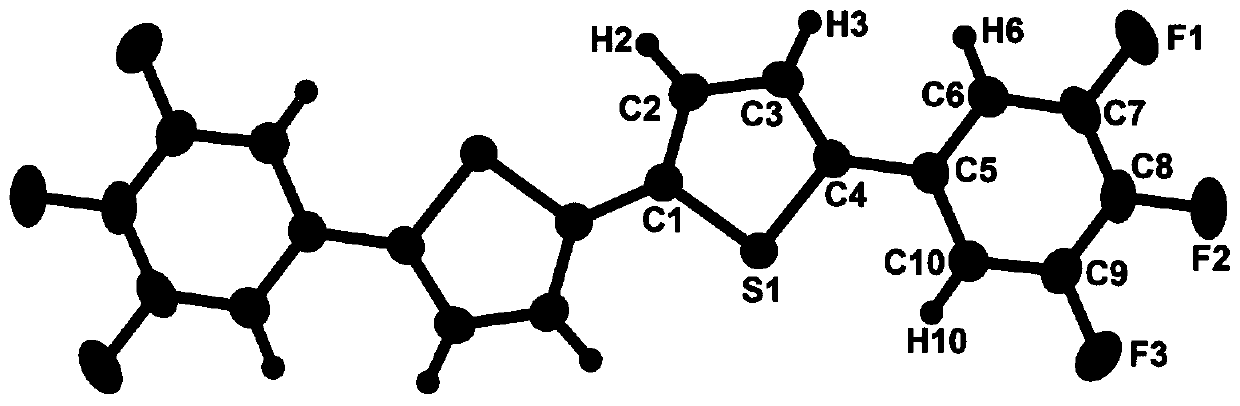
Trifluorophenyl-containing dithiophene derivative and preparation method thereof
A trifluorophenyl bisthiophene and trifluorophenyl technology are applied in the field of trifluorophenyl bisthiophene derivatives and their preparation, and can solve the problems affecting emission wavelength, luminous efficiency and service life, and electron cloud density of bisthiophene compounds. Changes, complex synthesis steps and other problems, to achieve the effect of favorable electronic transition and energy transfer, good photoelectric activity, and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Weigh 5,5-dibromo-2,2-dithiophene (0.324g1.0mmol), 3,4,5-trifluorophenylboronic acid (0.528g3.0mmol), tetrakis(triphenylphosphine)palladium (0.0462g0 .04mmol), salt of wormwood (2.08g15mmol) is mixed in the reaction device of 100mL, this reaction device is filled with nitrogen after vacuumizing, and repeats this operation three times, adds the mixed solution of 20mL toluene, 10mL ethanol, 2.0mL water after, in Under the protection of nitrogen, microwave reflux reaction for 2 hours; after the reaction solution is cooled, separate the liquids, extract with dichloromethane, combine the organic phases, wash with saturated brine and dry with magnesium sulfate to remove water; use a rotary evaporator to remove the solvent to obtain a solid powder , and then use pure petroleum ether as eluent to carry out column separation to obtain light yellow solid powder, which is recrystallized in petroleum ether to obtain light yellow blocky crystals.
Embodiment 2
[0023] Weigh 5,5-dibromo-2,2-dithiophene (0.162g0.5mmol), 3,4,5-trifluorophenylboronic acid (0.176g1.0mmol), tetrakis(triphenylphosphine)palladium (0.0578g0 05mmol), salt of wormwood (0.690g5mmol) is mixed in the reaction device of 100mL, this reaction device is filled with nitrogen after vacuumizing, and repeats this operation three times, then adds the mixed solution of 10mL toluene, 5.0mL ethanol, 1.0mL water, Under the protection of nitrogen, microwave reflux reaction for 4 hours; after the reaction solution was cooled, separate the liquids, extract with dichloromethane, combine the organic phases, wash with saturated brine and dry with magnesium sulfate to remove water; remove the solvent with a rotary evaporator to obtain a solid powder, and then use pure petroleum ether as eluent to carry out column separation to obtain a light yellow solid powder, which is recrystallized in petroleum ether to obtain a light yellow block crystal.
Embodiment 3
[0025] Weigh 5,5-dibromo-2,2-dithiophene (0.162g0.5mmol), 3,4,5-trifluorophenylboronic acid (0.211g1.2mmol), tetrakis(triphenylphosphine)palladium (0.0246g0 .03mmol), potassium carbonate (0.828g6.0mmol) was mixed in a 100mL reaction device, and the reaction device was vacuumized and filled with nitrogen, and the operation was repeated three times, and then a mixed mixture of 16mL toluene, 8mL ethanol, and 1.6mL water was added. Liquid, microwave reflux reaction under the protection of nitrogen for 3 hours; after the reaction solution is cooled, separate the liquids, extract with dichloromethane, combine the organic phases, wash with saturated brine and dry with magnesium sulfate to remove water; use a rotary evaporator to remove the solvent, A solid powder was obtained, and then purified petroleum ether was used as an eluent for column separation to obtain a light yellow solid powder, which was recrystallized in petroleum ether to obtain a light yellow block crystal.
[0026] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com