High-porosity dry powder inhaler carrier and supersaturated synthesis method and applications thereof
A dry powder inhaler, high porosity technology, used in medical preparations containing active ingredients, powder delivery, aerosol delivery, etc., can solve the problems of crystalline drug distribution, unevenness, poor absorption, etc. Controllable, improved uniformity, and high practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
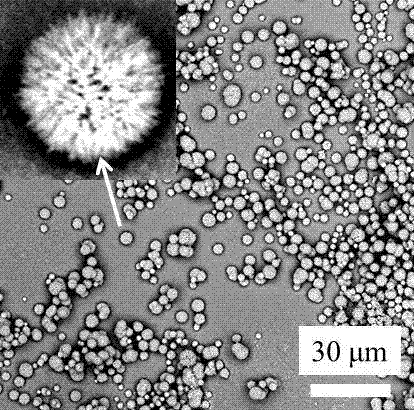
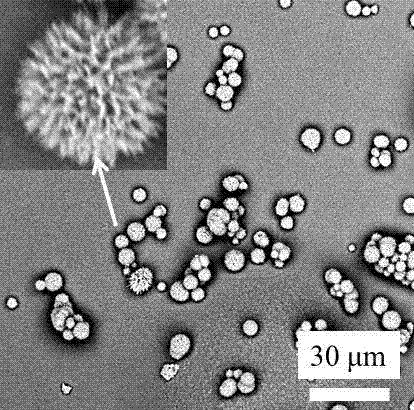
[0037] This embodiment provides a high-porosity dry powder inhaler carrier, which is a flower-shaped porous dry powder particle with a rough surface. The median diameter of a single particle under the electron microscope is 0.5-50 microns (controlled by the parameters of the preparation method), and the drug molecule The carrying capacity is 5-50% mass fraction, and the way of carrying drug molecules includes physical contact adsorption, micropore capillary action adsorption, and intermolecular force adsorption; the N of the high-porosity dry powder inhaler carrier before carrying drug molecules 2 The adsorption specific surface area is 10-100 square meters per gram, and the BET pore size distribution range includes micro (50 nanometers); the high-porosity dry powder inhaler carrier is in N after loading drug molecules 2 The specific surface area of adsorption decreases to 1-50% of the original value, and the change of microscopic morphology is mainly reflected in the decrea...
Embodiment 2
[0046] This embodiment provides a method for preparing a high-porosity dry powder inhaler carrier, comprising the following steps:
[0047] S1: Mix 50 grams of lactose with 50 grams of water (1:1 mass fraction ratio);
[0048] S2: Heating the mixed solution to 99 degrees, stirring and dissolving to obtain a 1g / g lactose solution;
[0049] S3: Quickly add high-temperature lactose solution to 1L ethanol solvent at 20 degrees (1:20 volume fraction ratio);
[0050] S4: after standing at room temperature for 5 minutes, centrifuge to separate the solid, and dry it with wind at room temperature.
Embodiment 3
[0052] This embodiment provides a method for preparing a high-porosity dry powder inhaler carrier, comprising the following steps:
[0053] S1: Mix 10 grams of lactose with 100 grams of water (mass fraction ratio of 1:10);
[0054] S2: spray drying at 150 degrees to prepare lactose supersaturated solid phase sample particles;
[0055] S3: quickly add lactose particles into 400mL ethanol solvent at 25 degrees (mass fraction ratio of 1:40);
[0056] S4: The solid was separated after standing at room temperature for 30 minutes, and dried at 40°C for 2 hours to constant weight.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com