Method for separation and determination of two medicine contents and related substances in tazarotene-betamethasone cream
A technology of tazarotene and betamethasone, applied in the field of analytical chemistry, to achieve accurate, reliable and repeatable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Method for Separating and Determining Related Substances in Tazarotene Betamethasone Cream
[0048] Instrument: SHIMADZU LC-20A (Shimadzu, Japan)
[0049] Chromatographic column: VP-ODS 4.6×250mm 5μm
[0050] Mobile phase A: 0.1% trifluoroacetic acid solution-methanol (95:5)
[0051] Mobile Phase B: Acetonitrile
[0052] Flow rate: 1.0ml / min
[0053] Detector: UV
[0054] Detection wavelength: 250nm
[0055] Injection volume: 20μl
[0056] Chromatography Workstation: LabSolution
[0057] Linear gradient elution program:
[0058]
[0059]Sample preparation: Take about 5g of this product, accurately add 25ml of acetonitrile, heat in a water bath at 50°C, stir to melt and disperse the paste, continue to stir for 2 minutes, take it out, transfer it to a 50ml centrifuge tube with stopper while it is hot, and place it on ice Cool in a water bath for more than 30 minutes, centrifuge to separate the layers, take the supernatant and filter it quickly, take t...
Embodiment 2
[0060] Embodiment 2 The method for separating and measuring the content of two kinds of drugs in tazarotene betamethasone cream
[0061] Instrument: SHIMADZU LC-20A (Shimadzu, Japan)
[0062] Chromatographic column: VP-ODS 4.6×250mm 5μm
[0063] Mobile phase A: 0.1% trifluoroacetic acid solution-methanol (95:5)
[0064] Mobile Phase B: Acetonitrile
[0065] Flow rate: 1.0ml / min
[0066] Detector: UV
[0067] Detection wavelength: 250nm
[0068] Injection volume: 20μl
[0069] Chromatography Workstation: LabSolution
[0070] Linear gradient elution program:
[0071]
[0072] Sample preparation: Take 2.0g of this product (approximately equivalent to 1mg of tazarotene and 1mg of betamethasone), weigh it accurately, put it in a 50ml brown measuring bottle, add 25ml of acetonitrile, heat it in a water bath at 50°C, and shake to make the main Dissolve the drug, let it cool to room temperature, and dilute to the mark with acetonitrile, shake well, filter, accurately measur...
Embodiment 3
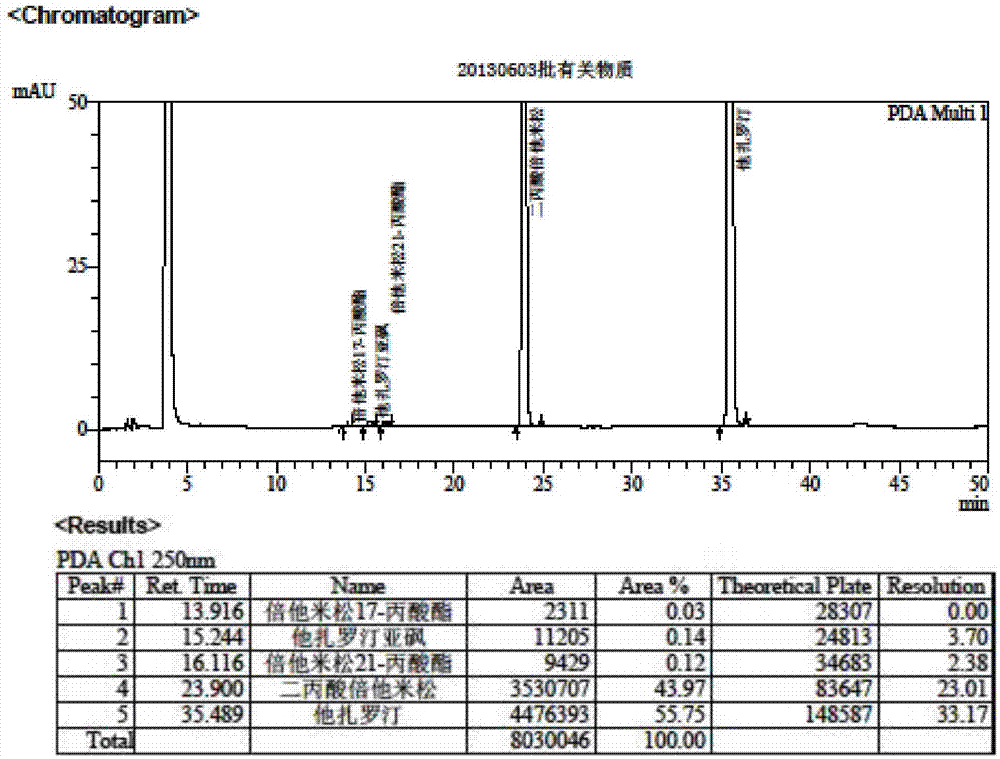
[0073] Embodiment 3 sample detection result
[0074] According to the methods of Example 1 and Example 2, the related substances and contents of multiple batches of tazarotene betamethasone cream were detected.
[0075] Table 8 Test results of related substances in tazarotene betamethasone cream
[0076] batch number Betamethasone 17-propionate, % Betamethasone 21-propionate, % Tazarotene sulfoxide, % 2010003 0.10 0.22 0.44 2010004 0.13 0.24 0.46 2010005 0.14 0.23 0.48 20130601 0.07 0.35 0.25 20130602 0.07 0.33 0.28 20130603 0.07 0.26 0.25
[0077] Table 9 Tazarotene betamethasone cream content test results
[0078] batch number Betamethasone dipropionate (calculated as betamethasone), % Tazarotene, % 2010003 100.5 100.6 2010004 99.9 100.3 2010005 97.9 99.9 20130601 98.2 97.0 20130602 97.8 96.6 20130603 99.6 96.8
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Snr | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com