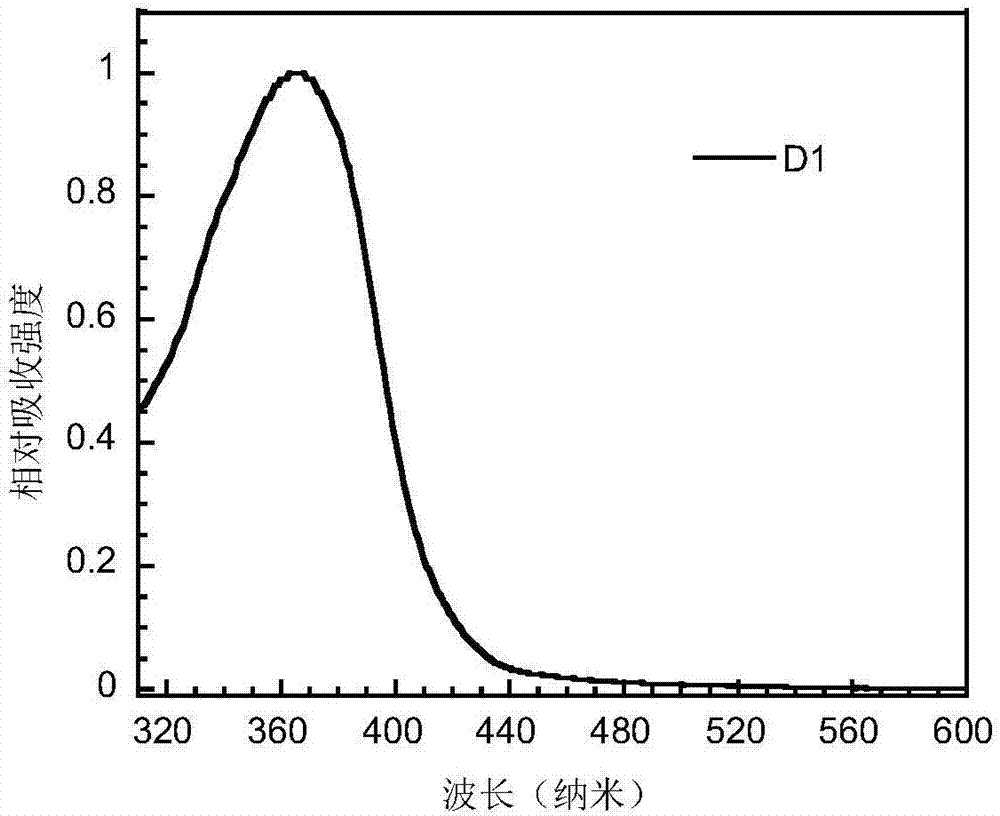
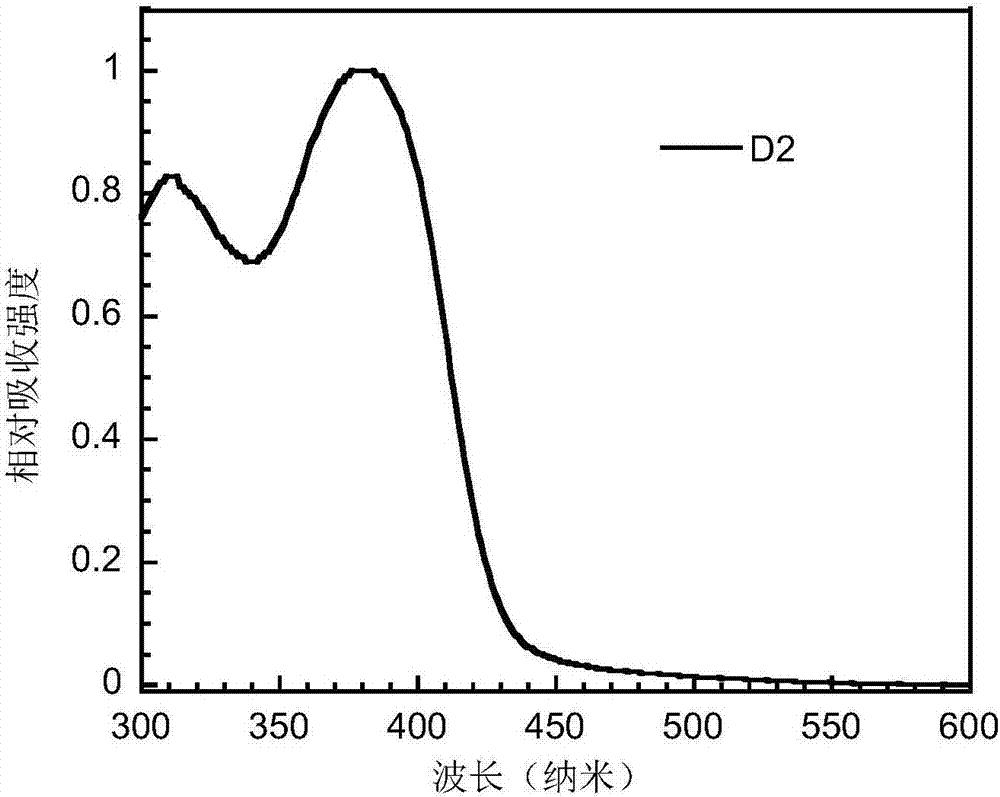
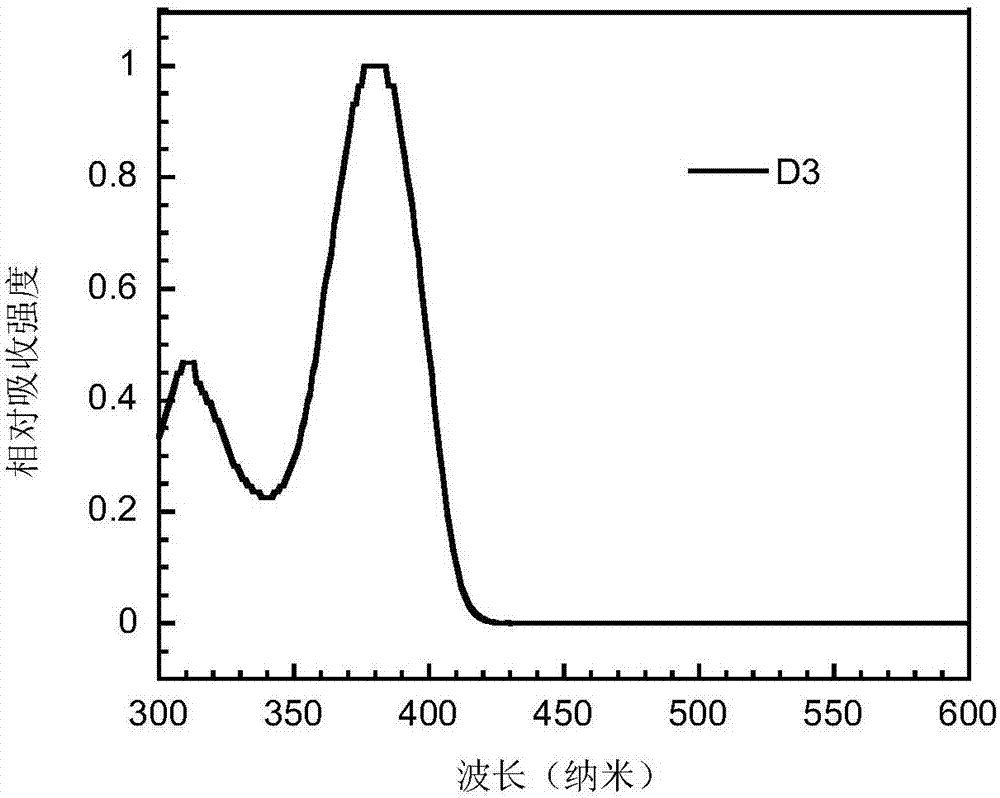
Bipolar small-molecular luminescent material based on heteroaromatic ring-fused-3-S,S-dioxodibenzothiophene unit, and preparation method and application thereof
A technology of dioxydibenzothiophene and light-emitting materials, which is applied in the directions of light-emitting materials, electrical components, chemical instruments and methods, etc., achieves the effects of simple preparation process, improved material device efficiency, and good electron and hole transport performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Methyl 1-bromo-2-quinoxalinecarboxylate
[0037] Under an argon atmosphere, add 1-bromo-2-quinoxalinecarboxylic acid (10g, 39.83mmol) into a two-necked flask, add 100mL of methanol, then add concentrated sulfuric acid (39.06mg, 398.29umol) dropwise, and heat to 110 °C, reacted for 18h. The reaction mixture was poured into water, extracted with ethyl acetate, and the organic layer was washed with brine and dried over anhydrous magnesium sulfate. After the solution was concentrated, a white solid crude product was obtained, which was purified by silica gel column chromatography (petroleum ether / dichloromethane=3 / 1, v / v was selected as the eluent), and the product was placed in the refrigerator for a long time to obtain a white solid with a yield of 85%. . 1 H NMR, 13 The results of CNMR, MS and elemental analysis showed that the obtained compound was the target product. The chemical reaction equation is as follows:
[0038]
Embodiment 2
[0040] Methyl 1-boronate-2-quinoxalinecarboxylate
[0041]Under argon atmosphere, the compound 1-bromo-2-quinoxalinecarboxylic acid methyl ester (10g, 37.72mmol) was dissolved in anhydrous tetrahydrofuran (THF), stirred at -78°C for 20 minutes, then added n-butyl Lithium (21.05g, 113.16mmol), stirred at -78°C for 2 hours, then added isopropoxy pinacol ester (9.66g, 150.88mmol), stirred at -78°C for 1 hour, and reacted at room temperature for 16 hours . The reaction mixture was poured into water, extracted with ethyl acetate, and the organic layer was washed with brine and dried over anhydrous magnesium sulfate. After the solution was concentrated, a white solid crude product was obtained, which was purified by silica gel column chromatography (petroleum ether / dichloromethane=2 / 1, v / v was selected as the eluent), and the product was placed in the refrigerator for a long time to obtain a white solid with a yield of 75%. . 1 H NMR, 13 CNMR, MS and elemental analysis results s...
Embodiment 3
[0044] Preparation of 3-bromo-S,S-thiofluorene dioxide
[0045] (1) In a 150 mL round bottom flask, 5 g of biphenyl was dissolved in 80 mL of dichloromethane, 11.8 g of bromosuccinimide was added at room temperature, and then reacted at room temperature for 48 hours. After the reaction, the reactant was poured into water, extracted with dichloromethane, washed with water, dried over anhydrous magnesium sulfate, evaporated to remove the solvent, and then recrystallized with petroleum ether. 5.65 g of white solid was obtained, yield 75%.
[0046] (2) Add 20g of 4,4'-dibromobiphenyl to a 150mL three-necked flask, dissolve it in 50mL of chloroform, add 11.4mL of chlorosulfonic acid dropwise, keep the reaction system below 50°C, and react for 3 hours. After the reaction, the reactant was poured into 500mL of crushed ice, and the ice was melted with Na 2 CO 3 The solution was adjusted to be neutral, and the insoluble matter was filtered out, washed with water and dried, and then ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com