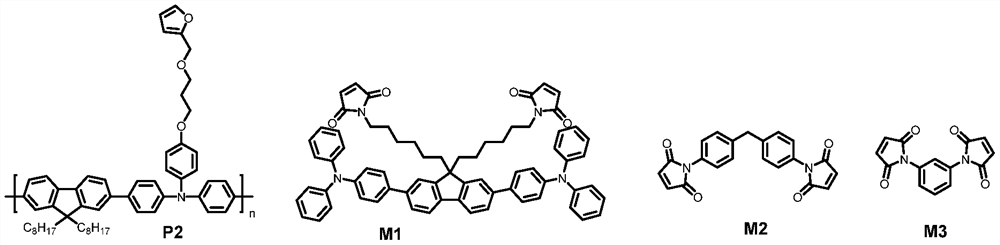
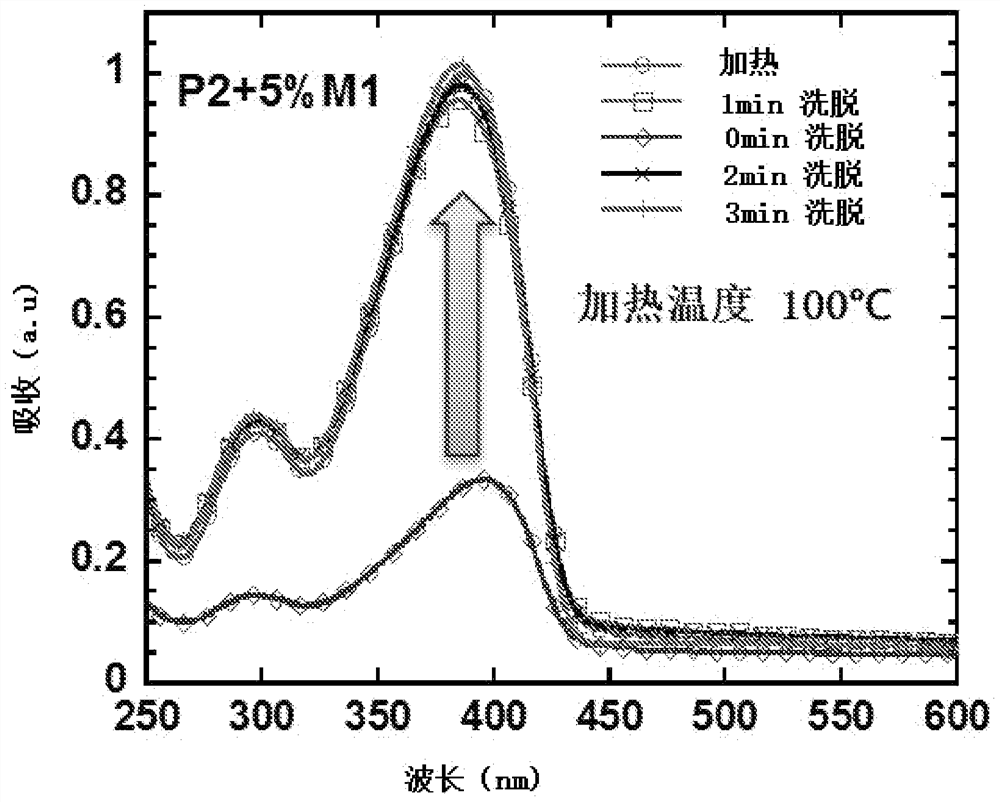
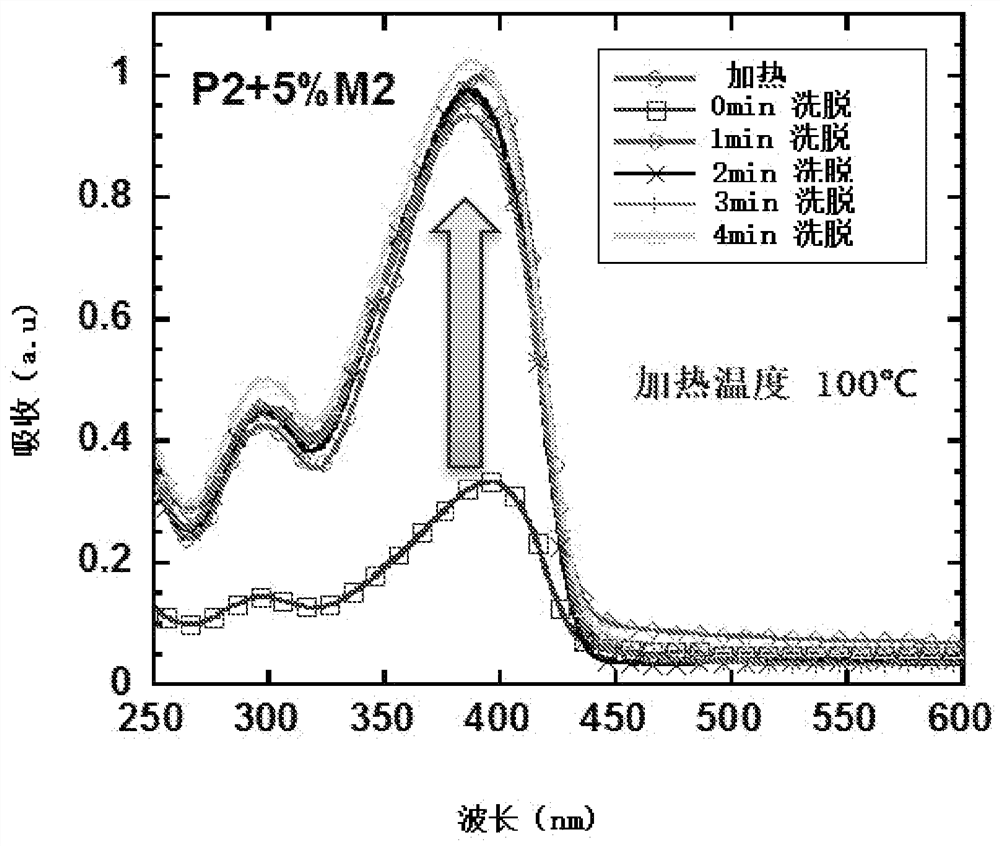
Crosslinkable polymers based on Diels-Alder reaction and their applications in organic electronic devices
An organic electronic device, polymer technology, applied in the direction of electric solid devices, electrical components, semiconductor devices, etc., can solve the problem of device life to be improved, and achieve excellent solvent resistance, good cross-linking effect, and short time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0257] Example 1: Synthesis of polymer P1 containing conjugated diene functional group D
[0258]
[0259] Synthesis of 2,5-diphenyl-p-xylene (3):
[0260] Add 26.40g (0.1mol) of 2,5-dibromo-p-xylene and 24.39g (0.2mmol) of phenylboronic acid into a 250ml three-neck round bottom flask, add 250ml of toluene, stir to dissolve, then add 50ml of water and 21.2g of Na 2 CO 3 (0.2mol), stir until the solids are all dissolved, add 0.5ml Aliquat 336 and 75mg triphenylphosphine tetrahelical palladium catalyst (o) (PPh 3 ) 4 Pd, nitrogen protection for 10min, then heating to reflux (92-100°C), reflux for 20min, turn off nitrogen to keep the system sealed, reflux reaction overnight, after cooling, extract the reaction solution with toluene (50mlx4), combine the organic phase, and sequentially use NaCl saturated solution Washed with water, distilled off the solvent and dried to obtain 22.48 g of white crystals, the theoretical value was 25.84 g, and the yield was about 87%. M.P.180...
Embodiment 2
[0283] Example 2: Synthesis of polymer P2 containing conjugated diene functional group D
[0284]
[0285] Synthesis of 2,7-Dibromofluorene (15)
[0286] Add fluorene (14) (100g, 602mmol) and iron powder (0.8g, 1.4mmol) into a 1-liter three-necked round-bottomed flask, add 500mL of chloroform to dissolve it completely, cool it to about 0-5°C in an ice-water bath, and slowly A mixture of liquid bromine (69 mL, 1337 mmol) and 100 mL of chloroform was added dropwise, protected from light, and after 1 hour of dropwise addition, reacted at room temperature for 10 hours, and a large amount of white solids precipitated. During the reaction process, the reaction was monitored by thin-layer chromatography. After the reaction was completed, saturated aqueous sodium bisulfite was added to remove excess unreacted liquid bromine. A large amount of white solid in the reaction mixture was filtered, the filtrate was washed three times with water, the oil layer was separated, concentrated,...
Embodiment 3
[0294] Example 3: Synthesis of Polymer P3 Containing Dienophile Functional Group A
[0295]
[0296] Synthesis of 4-Bromophenyl Acrylate (19)
[0297] Add 60% sodium hydride (3.68g, 91.6mol) to a tetrahydrofuran solution of p-bromobenzyl alcohol (16.3g, 87.3mol) under ice-cooling, react for 30min, then add acryloyl chloride (8.3g, 91.6mol), and the reaction continues Stir for 30min. Water was then added to terminate the reaction, and the organic solvent was removed by rotary evaporation. The residue was extracted with ethyl acetate, washed with saturated brine, and then mixed with silica gel powder to pass through the column. The ratio of ethyl acetate:petroleum ether was 80:20 as a rinse solution. 16 g of oil was obtained with a yield of 95%. 1 H-NMR (CDCl 3)δ: 6.03 (1H, dd, J = 10.5, 1.1Hz), 6.31 (1H, dd, J = 17.3, 10.5Hz), 6.61 (1H, dd, J = 17.3, 1.1Hz), 7.03 (2H, d , J=9.1 Hz), 7.50 (2H, d, J=9.1 Hz).
[0298] Synthesis of 4-(Diphenylamino)phenyl Acrylate (20)
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com