PH and anoxia dual-response tumor cell location naphthalimide rate fluorescent probe and synthesis method thereof
A ratiometric fluorescent probe, tumor cell technology, applied in the field of naphthalimide ratio fluorescent probes for localizing tumor cells with dual response to pH and hypoxia and their synthesis, which can solve the problems of limited accuracy of localizing tumor cells, etc. achieve the effect of improving accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 (synthesis of probe)
[0041] The reaction scheme is as follows:
[0042]
[0043] 1.1 Synthesis of compound 1:
[0044] Put 5.54g of A and 40mL of n-butylamine into a 250mL flask, add 100mL of absolute ethanol, reflux at 120°C for 3h. After cooling to room temperature, 8.0 g of white solid was obtained by suction filtration.
[0045] 1.2 Synthesis of Compound 2:
[0046]2.0 g of compound 1 and 3.2 mL of N-(2-aminoethyl)morpholine were added to a 100 mL flask and 0.01 mg of copper sulfate pentahydrate and 40 mL of ethoxyethanol were added. Under the protection of nitrogen, it was refluxed at 120°C for 8 h, spin-dried, and purified by column chromatography (DCM:methanol=10:1) to obtain 1.3 g of a yellow solid.
[0047] The H NMR spectrum of compound 2 is shown in Figure 7 .
[0048] 1.3 Synthesis of compound 3:
[0049] 130 mg NaH was dissolved in 2 mL THF, cooled to 0° C. under argon protection, 50 mg of compound 2 was dissolved in 3 mL THF and a...
Embodiment 2
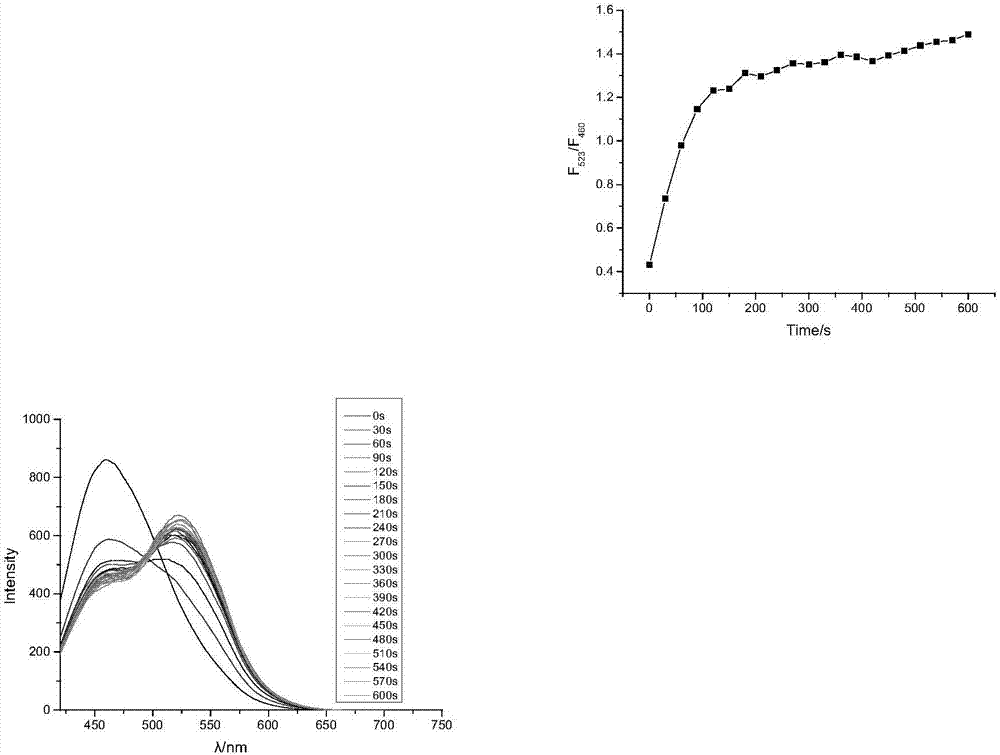
[0050] Embodiment 2 (the ultraviolet-visible absorption spectrum of compound 2 and compound 3)
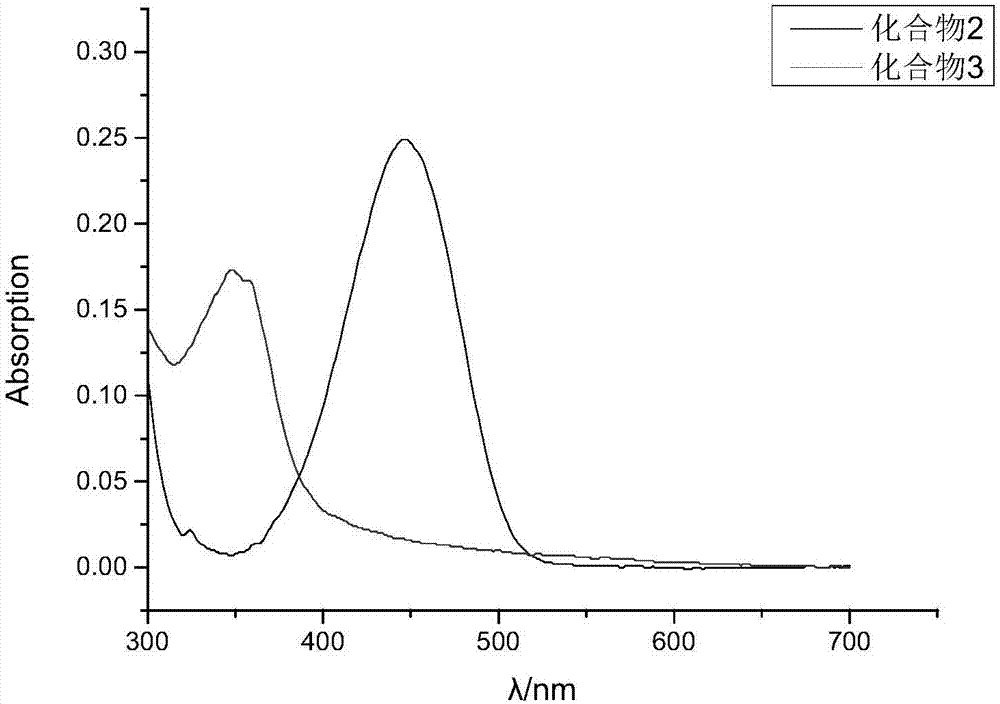
[0051] A DMSO solution with a concentration of 1 mM Compound 2 and Compound 3 was prepared. Take 3mL0.01M PBS buffer solution and add 30μL DMSO solution as blank control. Also take 3mL of the same PBS solution and add the prepared compound 2 and compound 3 solutions respectively, and test respectively to get figure 1 UV absorption curves of compound 2 and compound 3. Depend on figure 1 It can be seen that the isoabsorptive point of compound 2 and compound 3 is 389 nm.
Embodiment 3
[0052] Embodiment 3 (pH titration of compound 2)
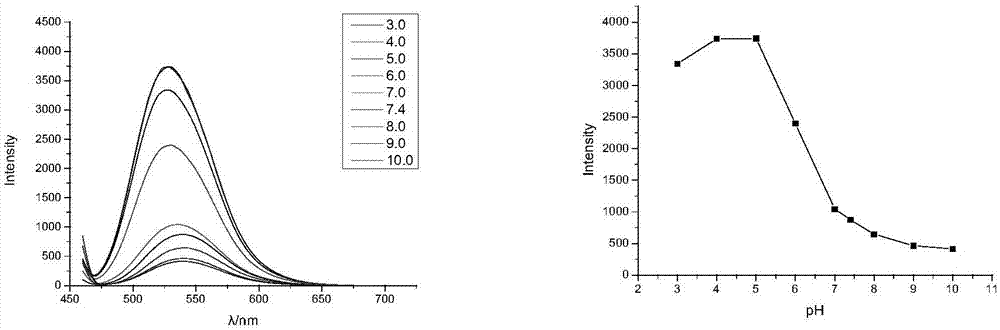
[0053] from figure 1 The excitation wavelengths for obtaining compound 2 were 449 nm. Take 3 mL of 0.01M PBS (pH7.4) buffer solution and add 30 μL of 1 mM compound 2 and compound 3 solutions respectively, and titrate with 0.1M HCl and NaOH solutions. The fluorescence intensities of compound 2 at pH 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, and 10.0 were collected respectively. Compound 2 measured results see figure 2 . Depend on figure 2 It can be seen that the titration transition of compound 2 is 4.0-7.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com