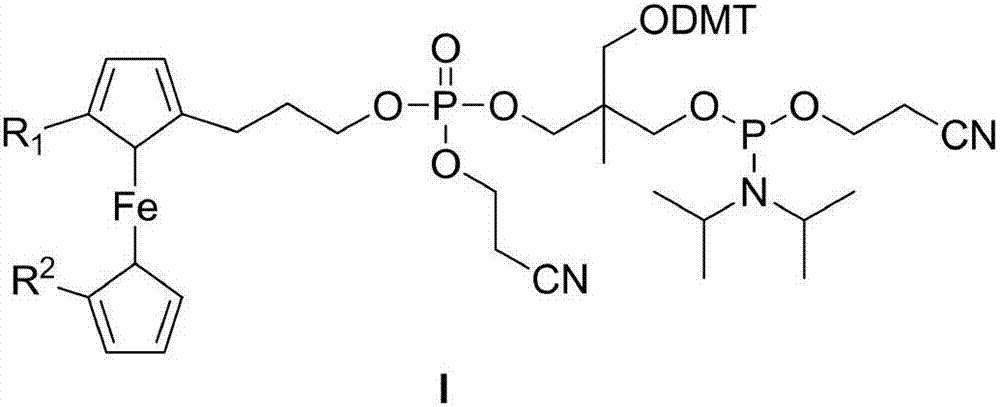
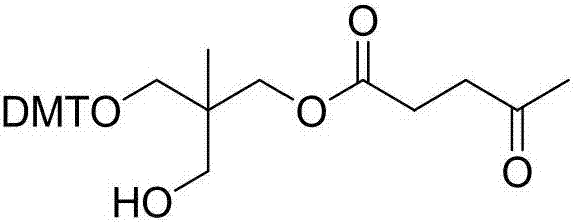
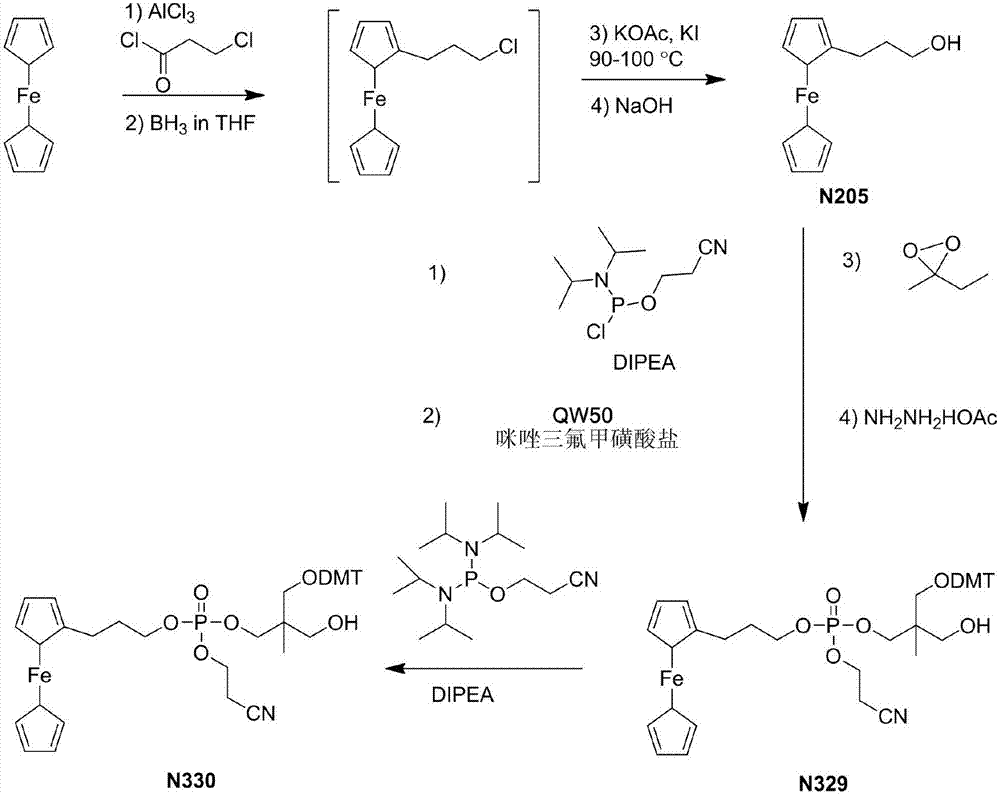
Preparation method of compound
A technology of compounds and complexes, which is applied in the field of preparation of ferrocene compounds, can solve the problems of expensive phosphoramidites, difficult quality control, and low reaction yields, and achieve rapid reactions, low production costs, and reproducibility Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080]
[0081] AlCl 3 (17.70g, 132.8mmol), DCM (200.0mL) in 500mL inclined two-necked flask, N 2 Under protection, lower to 0°C, slowly add 3-chloropropionyl chloride (13.5mL, 141.6mmol) dropwise, and keep warm for 1h to obtain A solution;
[0082] Take DMF (80.0mL) in a 100mL eggplant bottle, add NaBH in batches 4 (6.10g, 161.2mmol), heated to 45°C and stirred for 3h to obtain B solution;
[0083] Get ferrocene (20.00g, 107.5mmol) and be dissolved in DCM (52.0mL), N 2 Under protection, solution A was slowly added dropwise at room temperature, after the dropwise addition was completed, the reaction was carried out at room temperature for 30 minutes; when the temperature was lowered to 0°C, solution B was slowly added dropwise, after the addition was completed, it was raised to room temperature for 1 hour reaction. Slowly add water (200.0mL) to quench the reaction, separate layers, extract the aqueous phase with DCM, wash with water; anhydrous Na 2 SO 4 After drying an...
Embodiment 2
[0087]
[0088] Take THF (356.0mL), II-1 (24.00g, 200.0mmol), TsOH (1.20g, 6.3mmol) in a 1L oblique two-necked flask, slowly add benzaldehyde (21.4mL, 210.0mmol), dropwise , Reaction at room temperature for 12h.
[0089] Adjust the pH to 7.0 with ammonia water, evaporate THF under reduced pressure; 2 SO 4 After drying and concentrating, 37.12 g of white solid compound II-2 was obtained, with a yield of 89.1%.
Embodiment 3
[0091]
[0092] N 2 Under protection, take compound II-2 (5.00g, 24.0mol), DCC (6.43g, 31.2mmol), DMAP (0.293g, 2.4mmol), DCM (48.0mL) in a 100mL oblique two-necked flask, and drop to 0 ℃; Slowly add levulinic acid (2.7mL, 26.4mmol) dropwise and keep warm for 1h. Filtration and concentration gave blood red oily compound II-3.
[0093] Add MeOH (33.0mL) to the flask containing compound II-3 to dissolve, then transfer to a 500mL inclined two-neck flask, then add hydrochloric acid (33.2mL, 4mol / L), and react at room temperature for 2h.
[0094] Adjust pH to 7.0 with NaOH solution (33.2mL, 4mol / L), distill off MeOH, extract with DCM (3×40mL), anhydrous Na 2 SO 4 Drying and concentration gave brownish-red oily compound II-4.
[0095] N 2 Under protection, add TEA (3.3mL, 23.9mmol), DMAP (0.265g, 2.2mmol), DCM (27.7mL) to the flask containing compound II-4; slowly drop DMTCl dissolved in DCM (27.7mL) (6.65g, 23.9mmol) solution, reacted overnight.
[0096] Water (1×50mL), 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com