6-cyclohexyl methyl pyrimidone compounds (s-DACOs) non-nucleoside reverse transcriptase inhibitors (nnrtis) as well as preparation method and use thereof
An alkyl and alkoxy technology, used in organic chemistry, antiviral agents, etc., can solve problems such as toxic side effects, interfere with normal cell DNA chain growth, etc., and achieve the effect of low toxicity and good virus inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
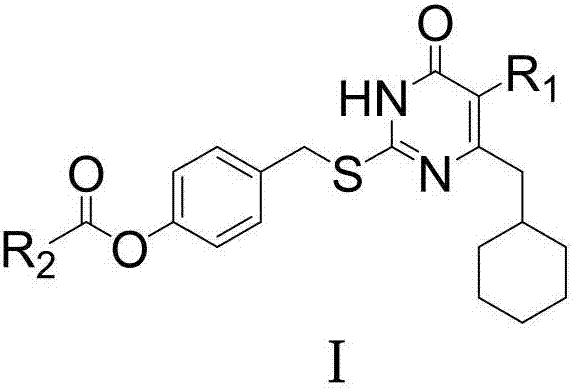
[0025] Example 1: Preparation of 5-alkyl-6-cyclohexylmethyl-2-(4'-carboxylate benzyl)thiopyrimidinone compound (I)
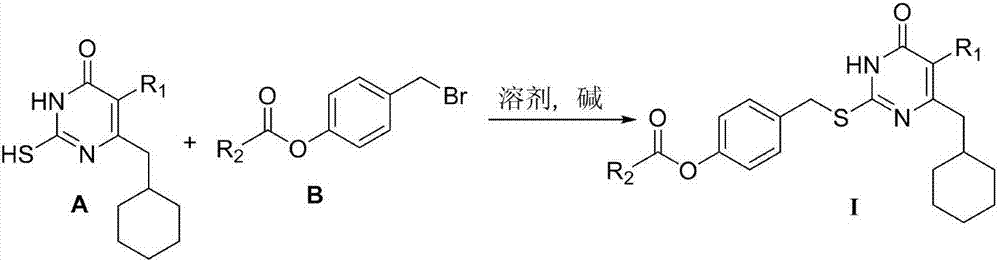
[0026] Dissolve 0.002mol of 6-cyclohexylmethyl-5-ethyl / isopropylthiopyrimidinone in 9ml of DMF, add 0.0024mol of anhydrous K 2 CO 3 , stirred at room temperature for 30 minutes, added 9ml of DMF dissolved with 0.0021mol 4-carboxycarboxybenzyl bromide (B) to dissolve, stirred at room temperature, stopped the reaction after TLC traced the disappearance of raw materials, poured the reaction solution into 80ml of ice water, filtered or used acetic acid Extract and concentrate with ethyl ester to obtain a crude product, which is purified by column chromatography or recrystallization.
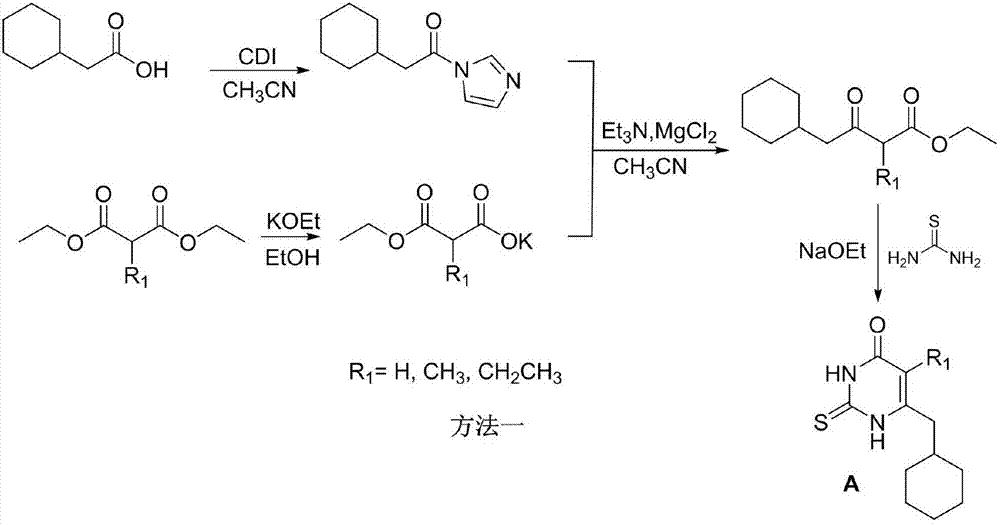
[0027] By the above-mentioned method, 6-cyclohexylmethyl-5-alkylthiopyrimidinone (A) and different 4-carboxycarboxybenzyl bromides (B) are reacted in a suitable solvent under alkali catalysis to obtain the general formula I The target compound, the structure and spectrum data of some...
Embodiment example 2
[0041] Implementation Case 2: Anti-HIV-1 Activity Test
[0042] C8166 cells infected with HIV-1 were used to test the anti-HIV biological activity at the cell level. The methods are described below.
[0043] Cytotoxicity test: The toxicity of the compound to C8166 cells was determined by MTT method. In a 96-well cell culture plate, the compound was diluted fivefold, and 4×10 5 / ml C8166 cell suspension. Three replicate wells were set up for each concentration. At the same time, a drug-free cell control and an AZT drug control were set. 37°C, 5% CO 2 Cultivate in an incubator for three days, add MTT solution to each well and incubate at 37°C for 4 hours. Then add 10% SDS-50% DMF to each well, 37°C, 5% CO 2 Incubate overnight in the incubator. After mixing, measure the OD value with BIO-TEK ELx800ELISA instrument (measurement wavelength: 595nm; reference wavelength: 630nm), draw the measurement reaction curve according to the experimental results, and calculate the CC 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com