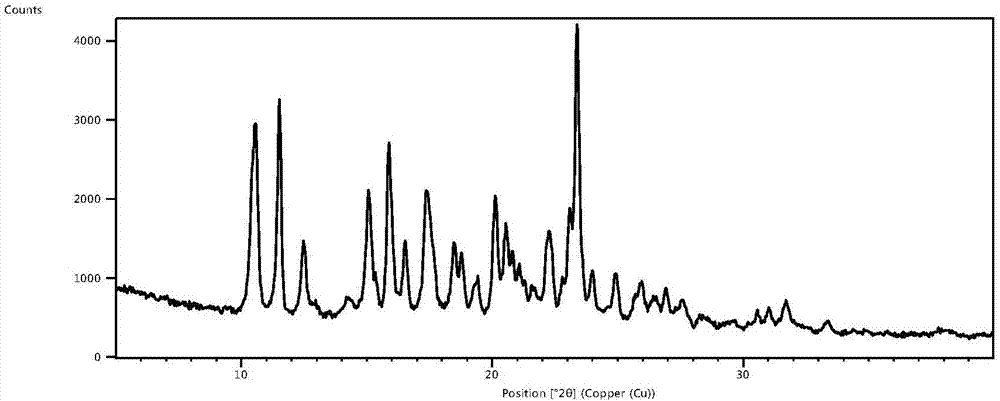
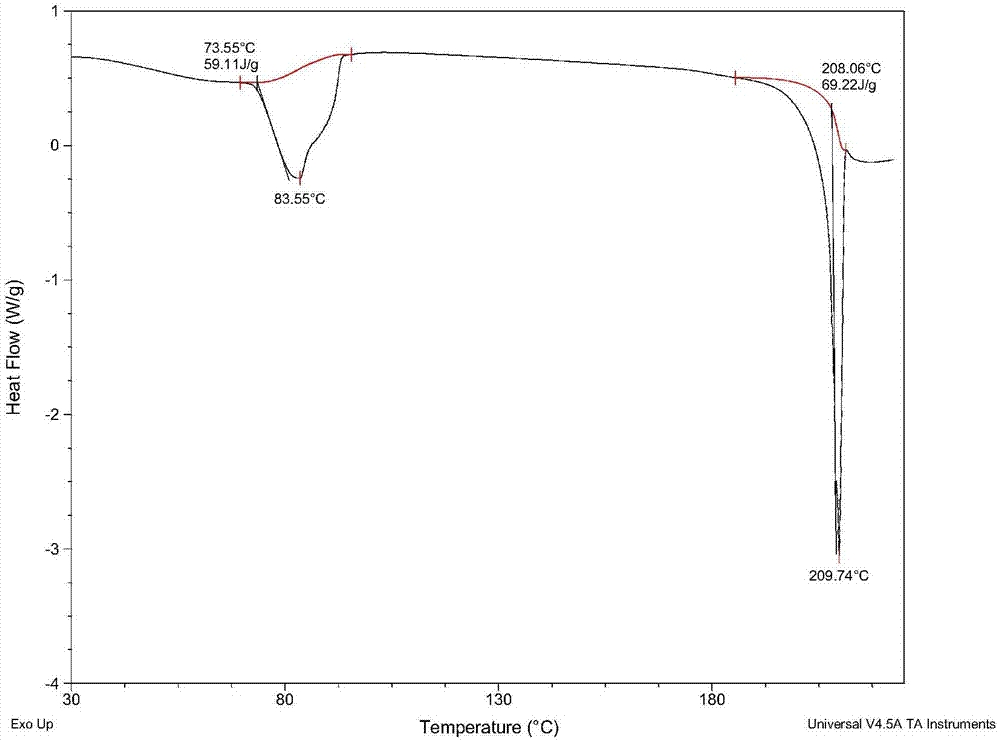
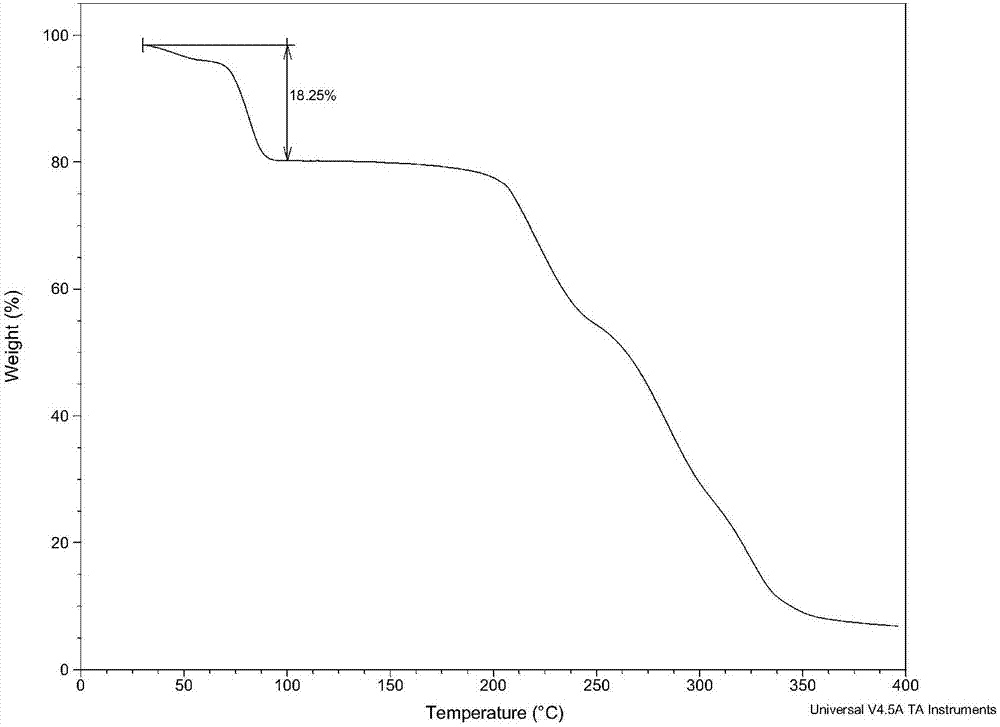
Glimepiride delta crystal form and preparation method thereof
A technology of glimepiride and crystal form, which is applied in the field of medicine and can solve problems such as low solubility, poor dissolution rate, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: Preparation method of glimepiride δ crystal form
[0071] Add 1.077 g of glimepiride crystal form I to 1.7 ml of N-methylpyrrolidone, stir at 120°C until completely dissolved, crystallize at -24°C for 48 hours, and separate by filtration to obtain glimepiride δ crystal form.
Embodiment 2
[0072] Example 2: Preparation method of glimepiride δ crystal form
[0073] Add 1.077g of glimepiride crystal form I to 1.7ml of N-methylpyrrolidone, stir at 110°C until completely dissolved, crystallize at -40°C for 36 hours, thaw at room temperature, filter and separate to obtain glimepiride δ crystal form.
Embodiment 3
[0074] Example 3: Preparation method of glimepiride δ crystal form
[0075] Add 1.077g of glimepiride crystal form I to 1.7ml of N-methylpyrrolidone, stir at 100°C until completely dissolved, crystallize at -80°C for 24 hours, thaw at room temperature, filter and separate to obtain glimepiride δ crystal form.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com