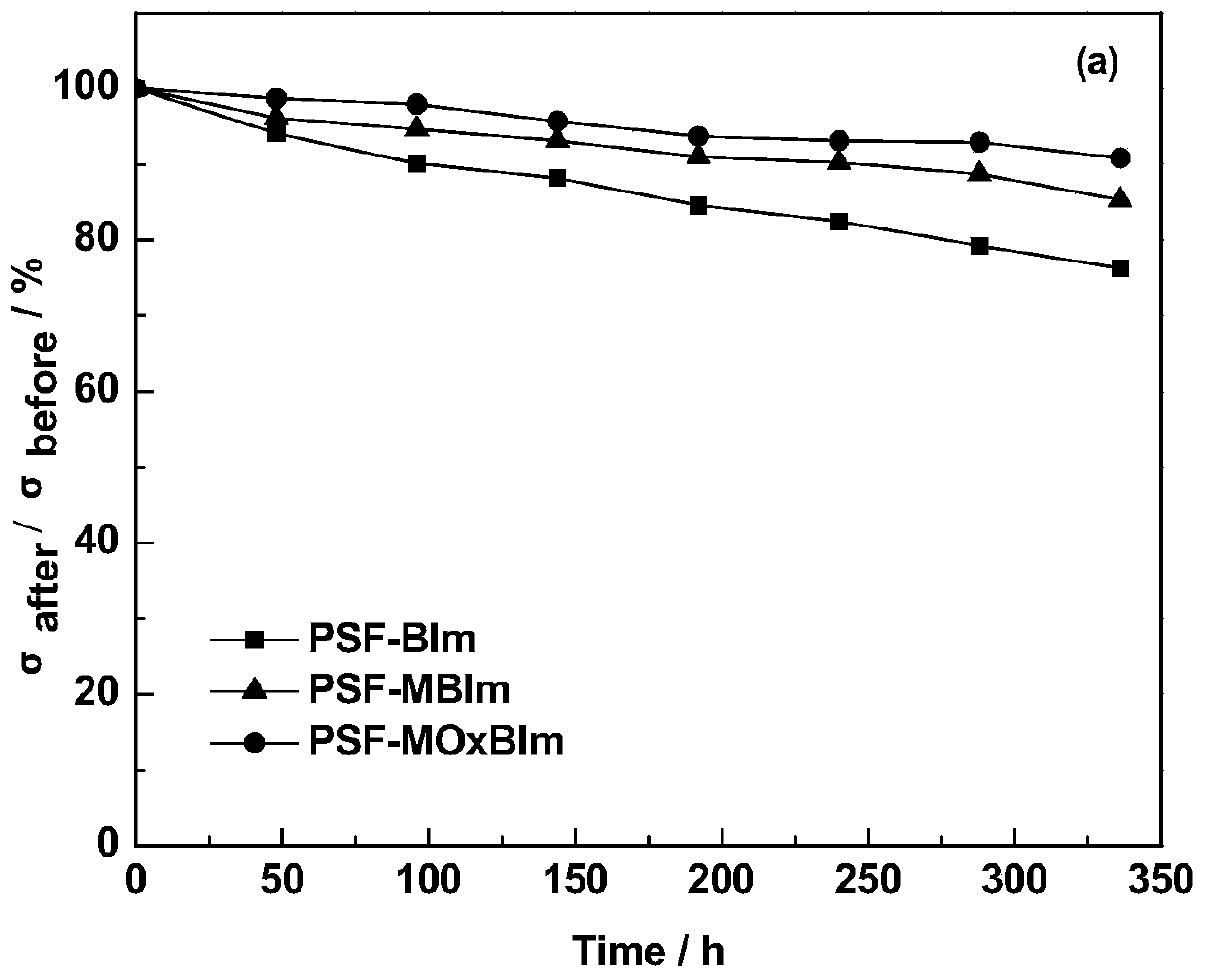
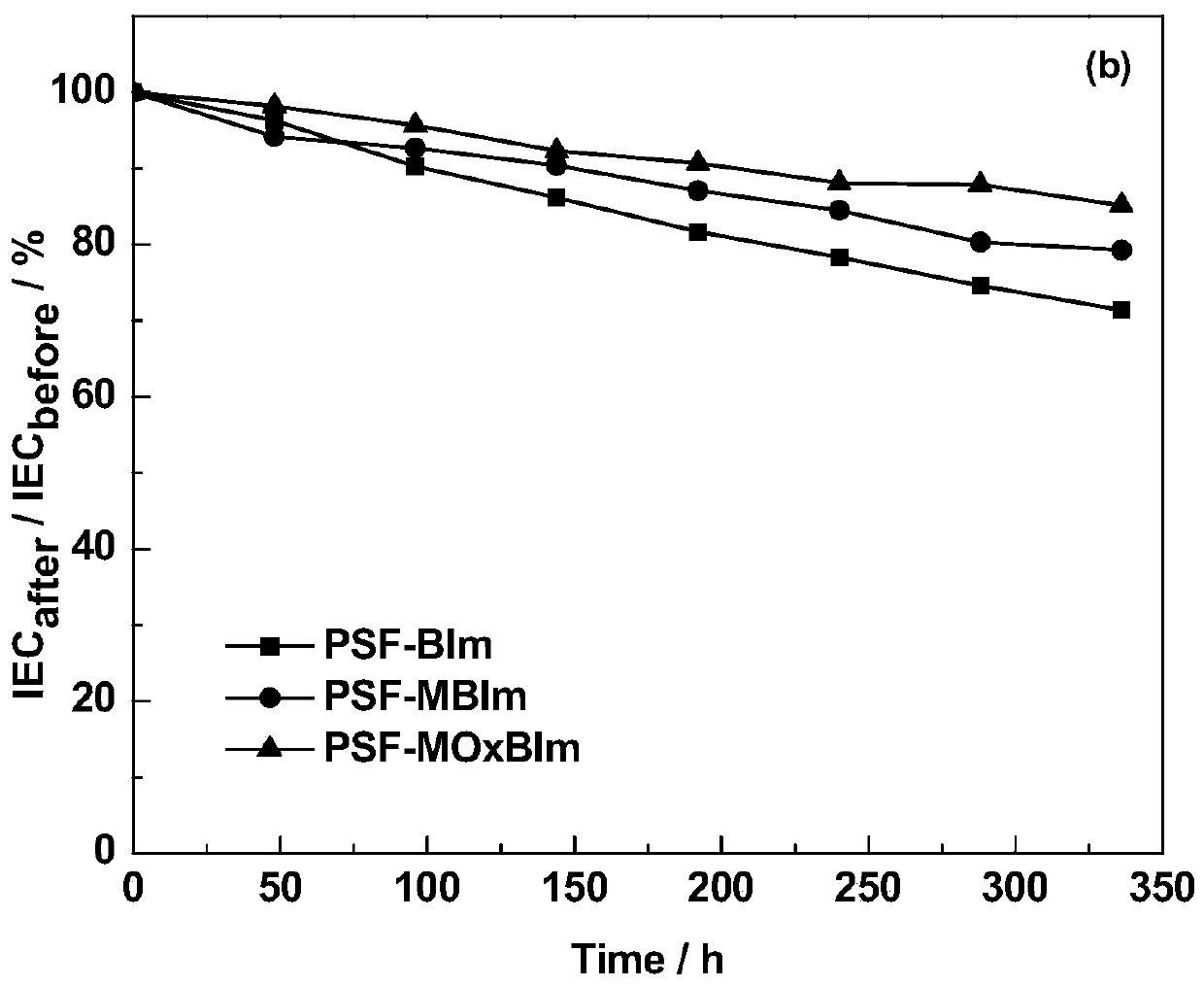
A kind of high base stable benzimidazole type alkaline anion exchange membrane and preparation method thereof
A basic anion and benzimidazole technology, which is applied in the field of high-alkaline stable benzimidazole anion exchange membrane and its preparation, can solve the problems of decreased conductivity and difficulty in meeting actual needs, and achieve mild conditions and good industrial application Foreground, the effect of simple operation of the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
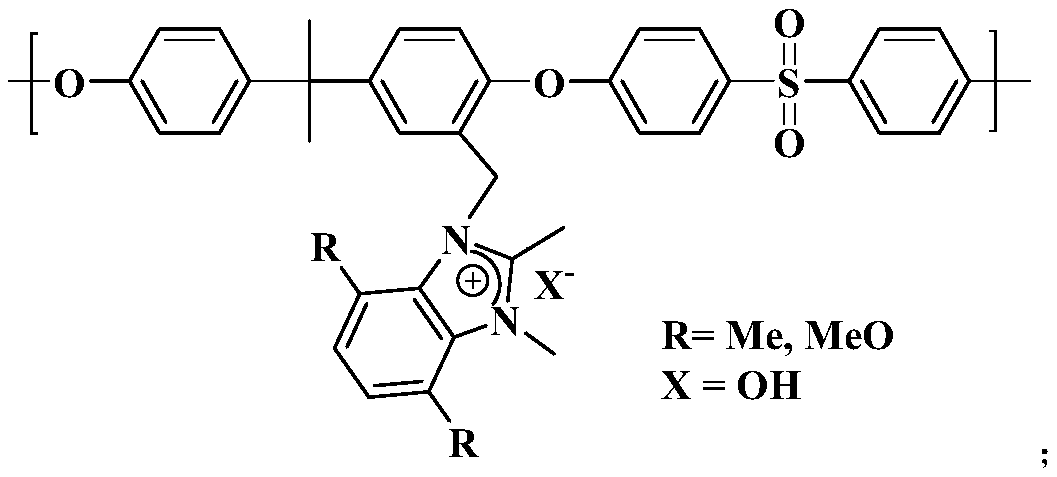
[0038] In this example, the anion exchange membrane based on 1.2-dimethylbenzimidazolium salt has the structure shown in the figure:
[0039]
[0040] The specific preparation method is:
[0041] Dissolve 0.5g of chloromethyl polysulfone with chloromethyl degree of 80% in 6.25ml of nitrogen methyl pyrrolidone, then add 1,2-dimethylbenzimidazole twice the amount of chloromethyl, and heat up to React at 80°C for 12 hours. After the reaction, pour it into 200ml of ethyl acetate, separate the precipitate, and dry it in vacuum for 24 hours to obtain a light yellow functionalized polymer.
[0042] The light yellow polymer obtained above was redissolved in 10 ml of nitrogen methyl pyrrolidone, centrifuged, poured into a glass watch glass and placed in an oven at 60° C. for 48 hours to obtain a polymer film with a thickness of 110 μm. Soak the membrane in 1mol / L KOH solution for 48 hours, and then fully wash it with distilled water until it becomes neutral to obtain an anionic pol...
Embodiment 2
[0045] In this example, the anion exchange membrane based on 1.2-dimethylbenzimidazolium salt has the structure shown in the figure:
[0046]
[0047] The specific preparation method is:
[0048] Dissolve 0.5 g of chloromethylated polysulfone with a chloromethyl degree of 100% in 6.25 ml of N,N-dimethylacetamide, and then add 1,2-dimethylbenzene 1.5 times the amount of chloromethyl and imidazole, heated to 90°C for 24 hours, after the reaction, poured into 200ml of acetonitrile, separated the precipitate, and dried in vacuum for 24 hours to obtain a light yellow functionalized polymer.
[0049] The light yellow polymer obtained above was redissolved in 10ml of N,N-dimethylacetamide, centrifuged, poured into a watch glass and placed in a 60°C oven for 48 hours to obtain a polymer film with a thickness of 108um. Soak the membrane in 1mol / L KOH solution for 48 hours, and then fully wash it with distilled water until it becomes neutral to obtain an anionic polymer membrane.
...
Embodiment 3
[0052] In this example, the anion exchange membrane based on 4,7-dimethyl-1.2-dimethylbenzimidazolium salt has the structure shown in the figure:
[0053]
[0054] The specific preparation method is:
[0055] Dissolve 0.5 g of chloromethylated polysulfone with a chloromethyl degree of 80% in 6.25 ml of nitrogen methyl pyrrolidone, and then add 4,7-dimethyl-1,2-bis Tolylbenzimidazole was heated to 80°C for 12 hours. After the reaction, it was poured into 200ml of ethyl acetate, and the precipitate was separated and vacuum-dried for 24 hours to obtain a pink functionalized polymer.
[0056] The light yellow polymer obtained above was redissolved in 10 ml of nitrogen methylpyrrolidone, centrifuged, poured into a watch glass and placed in an oven at 60°C for 48 hours to obtain a polymer film with a thickness of 108 um. Soak the membrane in 1mol / L KOH solution for 48 hours, and then fully wash it with distilled water until it becomes neutral to obtain an anionic polymer membran...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com