Non-noble metal catalyst for fuel battery, preparation method of non-noble metal catalyst and fuel battery
A non-precious metal and fuel cell technology, applied in the field of electrochemical energy, can solve the problems of low catalytic activity, poor oxygen reduction reaction activity, poor electron transport characteristics, etc., and achieve high catalytic activity, high efficient oxygen reduction ability, and electronic conductivity Enhanced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The first embodiment of the present invention provides a method for preparing a non-noble metal catalyst for a fuel cell, comprising the following steps:
[0035] Preparation of oxygen-doped g-C 3 N 4 ;
[0036] Doping g-C with oxygen 3 N 4 Spinning solution is prepared with transition metal salt, and the spinning solution is subjected to electrospinning treatment to obtain a precursor sample; the precursor sample is subjected to high temperature treatment to obtain a non-precious metal catalyst.
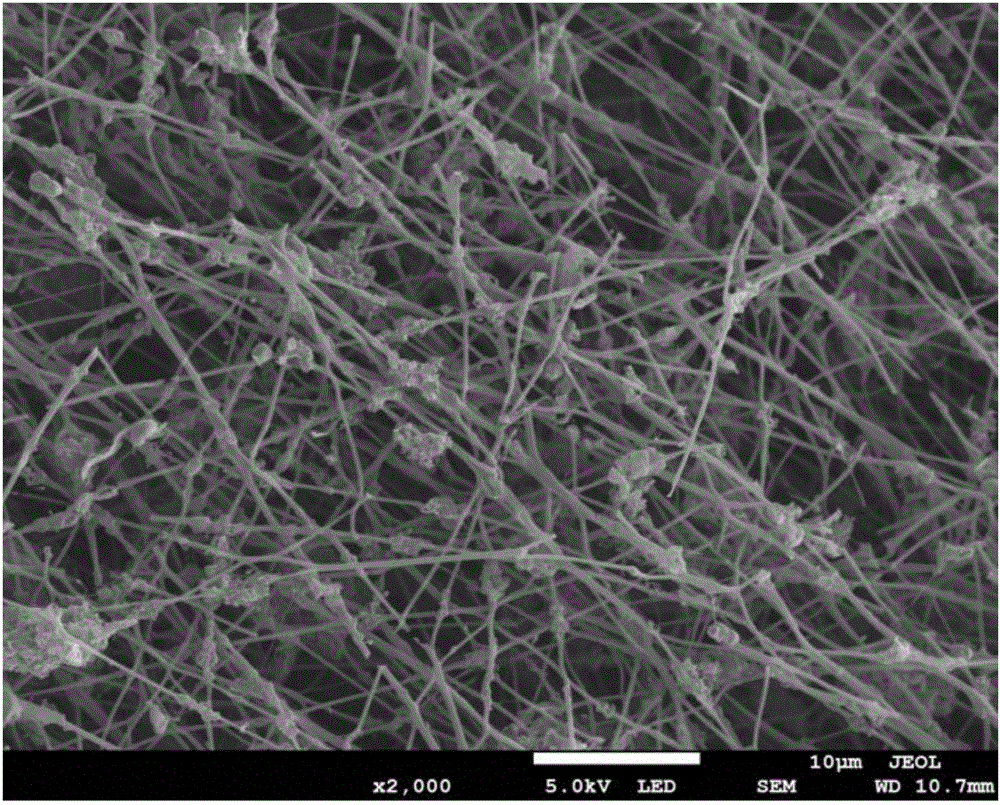
[0037] The preparation method of the fuel cell non-noble metal catalyst provided by the embodiment of the present invention first synthesizes oxygen-doped g-C 3 N 4 , using oxygen doping to change the g-C 3 N 4 electronic structure, so that g-C 3 N 4 The electronic conductivity is improved, the band gap is reduced, the top of the valence band is moved up, and the oxygen adsorption capacity is improved, so that the oxygen-doped g-C 3 N 4 It has a more efficient oxyg...
Embodiment 1
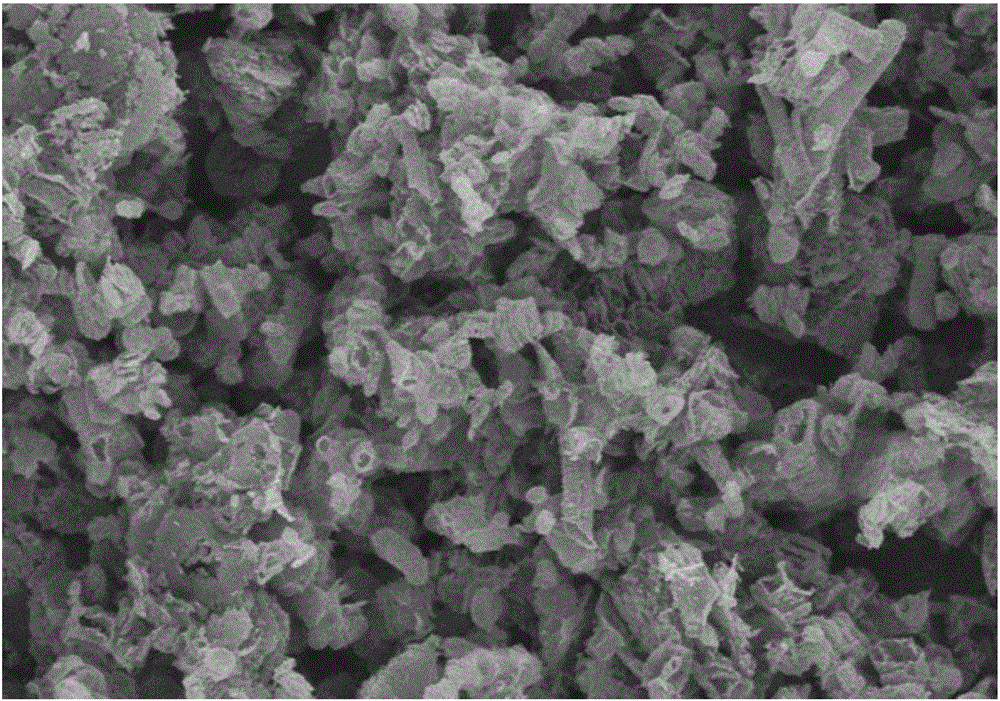
[0060] g-C 3 N 4 Catalyst: Accurately weigh 3g of melamine and 3g of cyanuric acid and disperse them in 100mL and 200mL of deionized water respectively. After heating to 80°C, the two are completely dissolved to form a transparent solution; 2 SO 4 Adjust the pH of cyanuric acid to 1, then quickly transfer the melamine solution to the cyanuric acid solution to generate a large amount of precipitate, and filter, wash, and dry the precipitate to obtain a precursor; the precursor is heated under the protection of Ar gas Polymerization, the heating program is as follows: first raise the temperature to 400°C, the heating rate is 10°C / min, keep it for 2 hours, then raise the temperature to 600°C, the heating rate is 10°C / min, keep it for 2 hours, and obtain g-C 3 N 4 catalyst. in, figure 1 For the g-C prepared in Example 1 3 N 4 SEM image of the catalyst. The specific surface area of the catalyst prepared by the above method is 38m 2 g -1 , under the three-electrode syste...
Embodiment 2
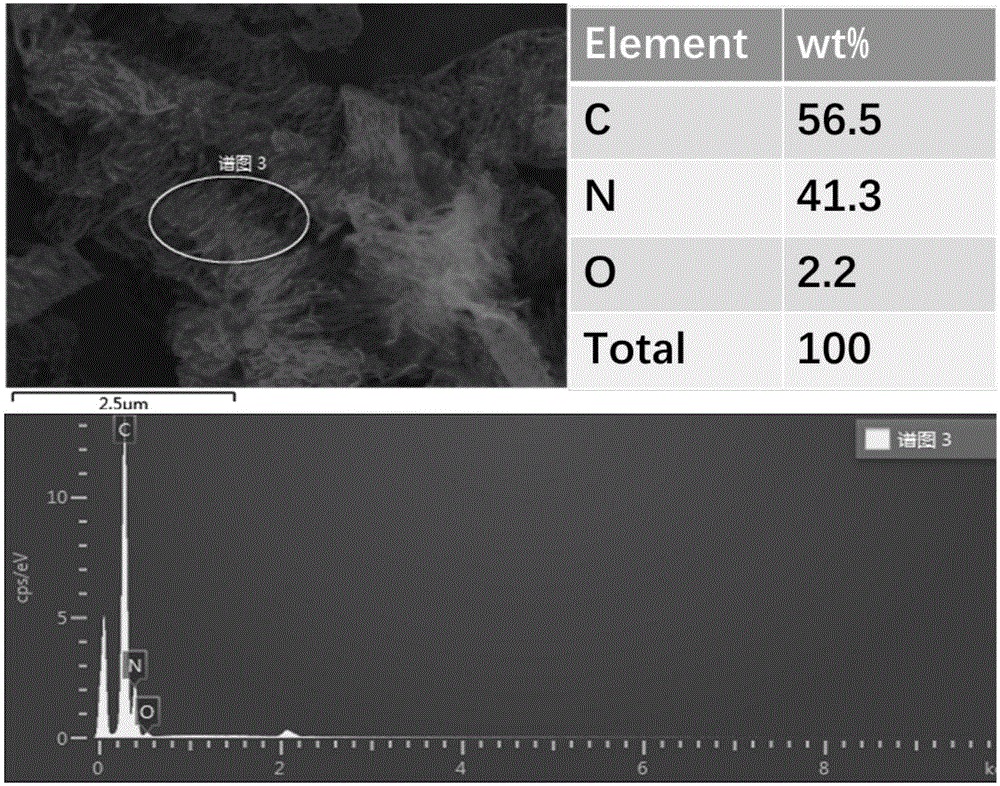
[0065] g-C 3 N 4 Catalyst: Accurately weigh 3g of melamine and 3g of cyanuric acid and disperse them in 100mL and 200mL of deionized water respectively. After heating to 80°C, the two are completely dissolved to form a transparent solution; 2 SO 4 Adjust the pH of cyanuric acid to 1, then quickly transfer the melamine solution to the cyanuric acid solution to generate a large amount of precipitate, and filter, wash, and dry the precipitate to obtain a precursor; the precursor is heated under the protection of Ar gas Polymerization, the heating program is as follows: first raise the temperature to 400°C, the heating rate is 10°C / min, keep it for 2 hours, then raise the temperature to 600°C, the heating rate is 10°C / min, keep it for 2 hours, and obtain g-C 3 N 4 catalyst. in, figure 2 For the g-C prepared in Example 2 3 N 4 Catalyst SEM image and EDS image. The specific surface area of the catalyst prepared by the above method is 38m 2 g -1 , under the three-electro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com