Zwitterionic polymer with reductive responding antitumor activity and synthesis thereof and application thereof as drug carrier
An anti-tumor activity, zwitterionic technology, applied in the preparation of anti-tumor drugs, in the field of zwitterionic polymers, to achieve the effects of reducing drug toxicity, good biocompatibility, and reducing damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
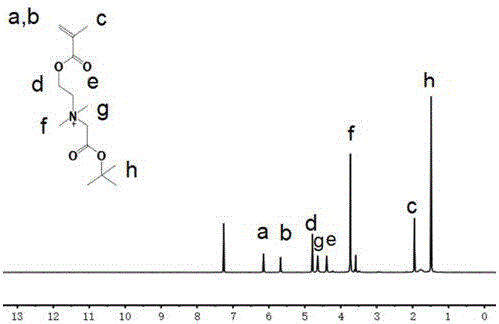
[0055] (1) Preparation of the hydrophilic monomer CBB-tBu: Weigh 1.57 (10 mmol) dimethylaminoethyl methacrylate and dissolve it in 10 mL of acetone. Add 5 mL of acetone solution containing 2.93 (15 mmol) tert-butyl bromoacetate, react for 6 hours, then filter the filtrate after the reaction, wash it with anhydrous acetone several times, and dry it in vacuum at 25°C for 24 hours to obtain a white solid that is Water monomer CBB-tBu, labeled as compound , and the yield was 92.5%.
[0056] 1 H NMR (400 MHz, CDCl 3 ):δ6.13(s,1H,=C H H),5.67(s,1H,=CH H ),1.95(s,3H,-C H 3 ),4.74(t,2H,-OC H 2 CH 2 -),4.38(t,2H-OCH 2 C H 2 -),3.73(s,6H,-N(C H 3 ) 2 ),4.63(s,2H,-C H 2 -OOC),1.48(s,9H,-(C H 3 ) 3 ).
[0057] (2) Preparation of intermediate compounds: 0.42 g (2.7 mmol) bis(2-hydroxyethyl) disulfide, 0.5 g (1.35 mmol) RAFT reagent (2-(dodecyl trithiocarbonate group )-2-methylpropionic acid, 0.33g (2.7 mmol) 4-dimethylaminopyridine, dissolved in a round-bottomed flas...
Embodiment 2
[0067] (1) compound , compound , compound The preparation, with embodiment 1;
[0068] (2) of the compound Preparation: weigh 0.2g compound (0.2g, 0.04mmol), 0.18g (0.65mmol) compound , into a Shlenck bottle, add 2 mL of DMF, heat and stir to dissolve, add 0.025g (0.15mmol) azobisisobutyronitrile when cooled to room temperature, vacuumize and inflate with nitrogen for 3 times, seal and react at 60°C for 24 h, Precipitation with ether gave a white powdery solid. Dissolve the above white solid in 5 mL of dichloromethane, slowly drop trifluoroacetic acid into it for hydrolysis for 2 h, and precipitate with ether three times to obtain the compound . The yield was 76.8%.
[0069] 1 H NMR (600 MHz, CDCl 3 )(ppm):δ4.309(t,2H,-S-S-CH 2 CH 2 -OH), 1.365 (-OCH 2 CH 2 CH 2 CH 2 CH 2 COO-),1.623(-OCH 2 CH 2 CH 2 CH 2 CH 2 COO-),2.286(-OCH 2 CH 2 CH 2 CH 2 CH 2 COO-),4.041(-O CH 2 CH 2 CH 2 CH 2 CH 2 COO-),3.73(s,6H,-N(C H 3 ) 2...
Embodiment 3
[0072] (1) compound , compound The preparation, with embodiment 2;
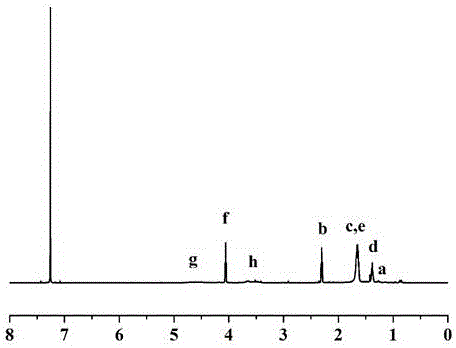
[0073] (2) compound Preparation: Under nitrogen protection, 0.5g (1 mmol) of the compound , 0.002g (0.05mmol) stannous octoate, 6.8 g (60 mmol) ε-caprolactone, dissolved in 10 mL toluene, polymerized at 110°C for 24 h, added 10 mL dichloromethane to dilute after the reaction, and then Add dropwise to 200 mL of cold methanol for precipitation under stirring, and wash several times with 200 mL of cold methanol, filter and dry to obtain 6.5 g of white solid which is the compound , and the yield was 89%.
[0074] 1 H NMR (600 MHz, CDCl 3 ) (ppm): δ0.859(t,C H 3 (CH 2 ) 10 CH 2 -S-(C=S)-S-),1.237(m, 20H,
[0075] CH 3 (C H 2 ) 10 CH 2 -S-(C=S)-S-),4.346(t,2H,-(C=O)OC H 2 CH 2 -),2.908(t,2H,-COOCH 2 C H 2 -),3.251(t,2H,-S-S-C H 2 CH 2 -OH), 4.309(t,2H,-S-S-CH 2 CH 2 -OH)1.365(-OCH 2 CH 2 CH 2 CH 2 CH 2 COO-),1.623(-OCH 2 CH 2 CH 2 CH 2 CH 2 COO-),2.286(-O...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com