Lipopeptid for restraining HIV with broad spectrum, derivatives, drug compound and application thereof
A derivative, HIV-2 technology, applied in the direction of microorganisms, biochemical equipment and methods, peptides, etc., can solve problems such as short biological half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
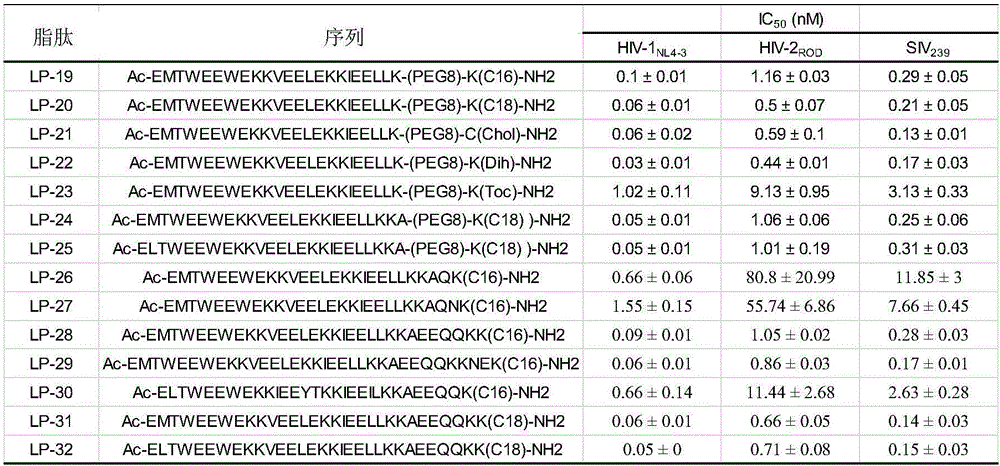
[0162] Embodiment 1, the design of lipopeptide
[0163] In this example, lipopeptides with the activity of inhibiting HIV-1 and / or HIV-2 and / or SIV are designed, and their names are LP-19, LP-20, LP-21, LP-22, LP-23, LP-24, LP-25, LP-26, LP-27, LP-28, LP-29, LP-30, LP-31 and LP-32, their structures are as figure 1 shown.
Embodiment 2
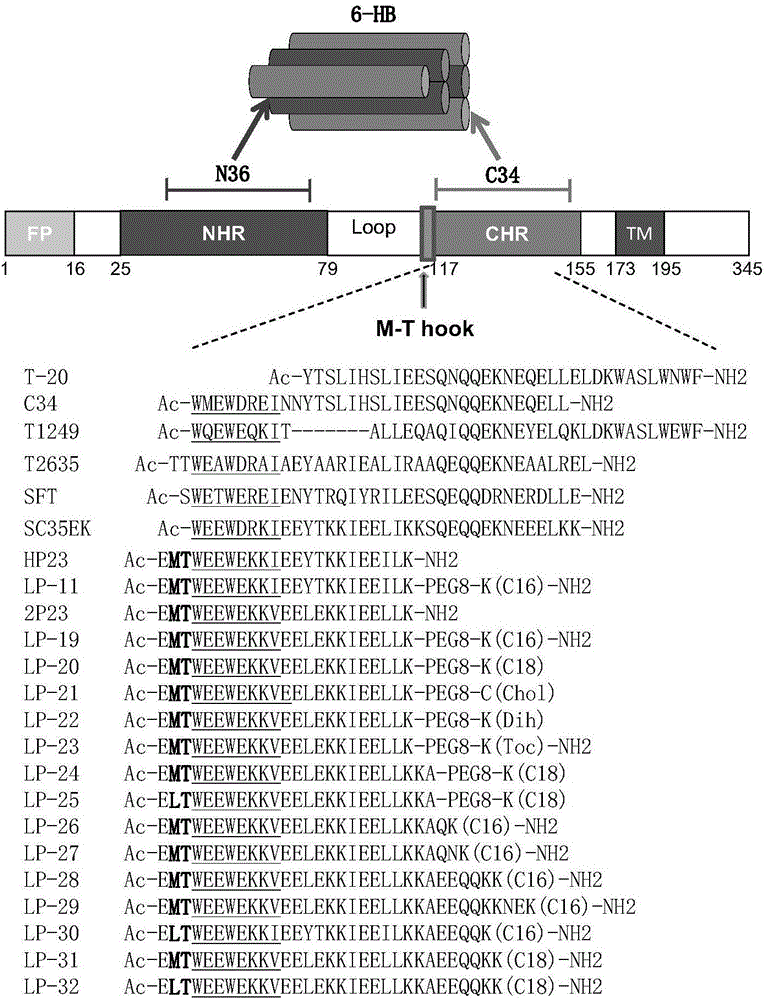
[0164] Embodiment 2, the preparation of polypeptide and lipopeptide
[0165] 1. Preparation of polypeptides
[0166] The amino-terminals of the polypeptides T-20 and 2P23 are connected with acetyl groups as the amino-terminal protecting group, and the carboxy-terminals are connected with amino groups as the carboxy-terminal protecting group. The structures of these polypeptides are as follows: figure 2 shown. These peptides were synthesized by standard solid-phase peptide synthesis method (Fmoc / tBu strategy), manually synthesized from the carboxy-terminus to the amino-terminus. All polypeptide sequences are amidated at the C-terminus and acetylated at the N-terminus according to the routine of peptide synthesis. Amino acid protected by N-fluorenylmethoxycarbonyl (Fmoc), Rink resin (substitution constant is 0.44mmol / g) is used as a solid phase carrier, and the amino protecting group is removed with a DMF solution of 25% (volume percentage) piperidine For Fmoc, each removal ...
Embodiment 3
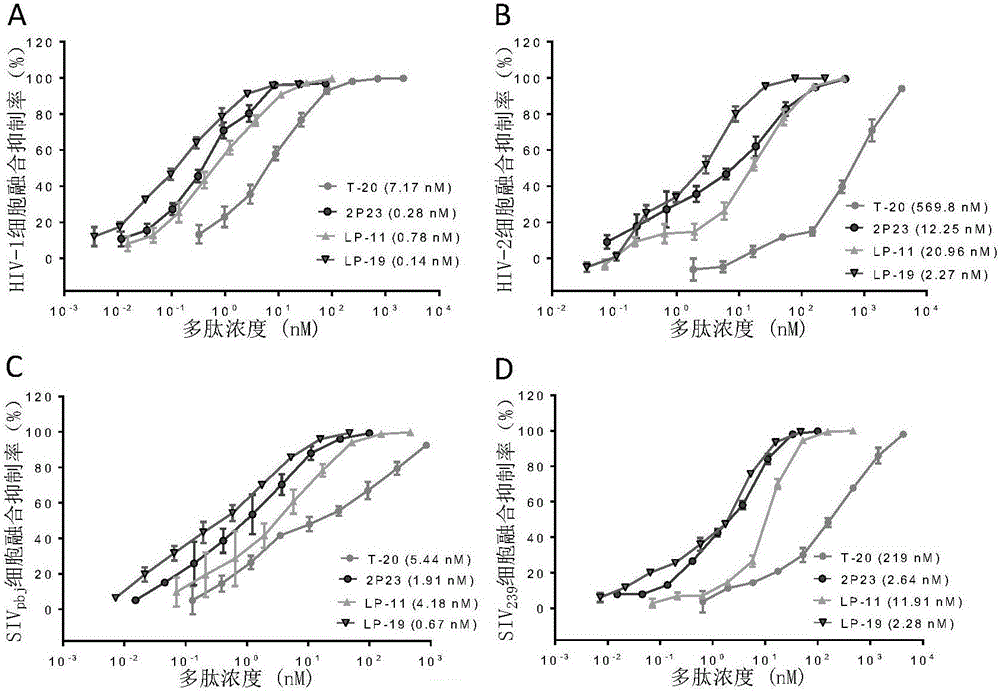
[0180] Example 3, detection of broad-spectrum antiviral activity of lipopeptide
[0181] 3.1 Experimental materials and methods
[0182] 3.1.1 Inhibitory effect of lipopeptide on cell fusion mediated by HIV and SIV
[0183] For the materials and methods of the HIV-1, HIV-2 and SIV-mediated cell fusion inhibition experiments, refer to the literature published by Xiong S et al. (Reference 13 in the Background Art). Among them, HEK293T cells (referred to as 239T cells) were purchased from the American Type Culture Collection (ATCC); U87CD4+CXCR4+ cells were provided by the American NIH AIDS Reagent and Reference Project (catalogue number: 4036); HIV-2 strain ROD molecular Cloned plasmid pROD was kindly provided by Prof. Nuno Taveira, University of Lisbon, Portugal; expresses SIV strain SIV pbj and SIV 239 The plasmids of the envelope protein (pSIVpbj-Env and pSIV239 respectively) were donated by Professor Xu Jianqing of Fudan University; the fluorescent reporter system plasmid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com