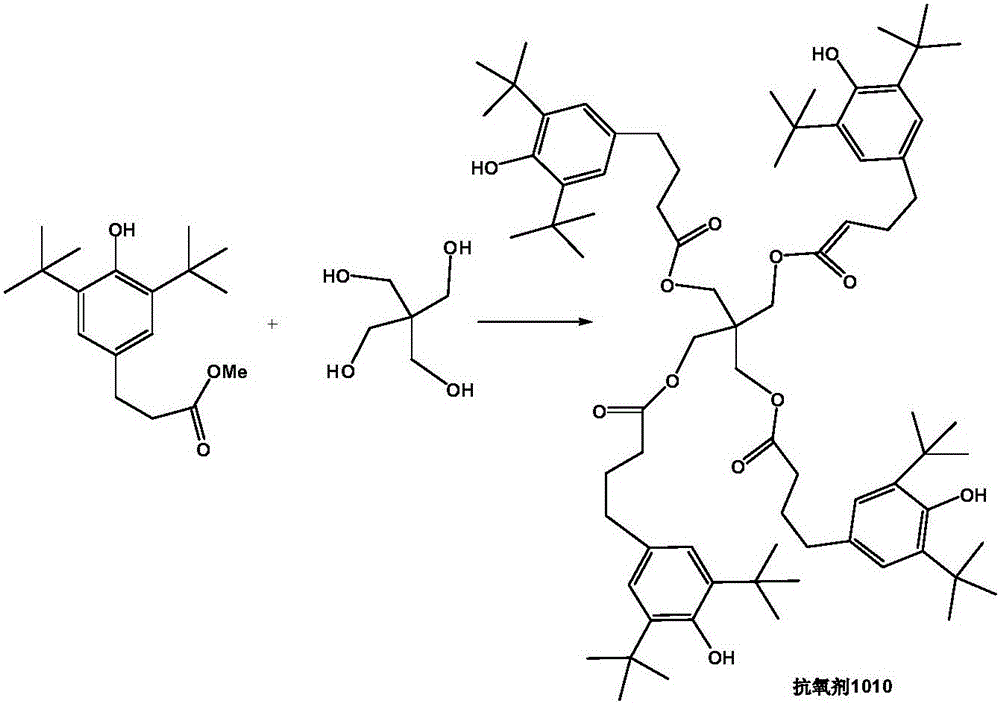
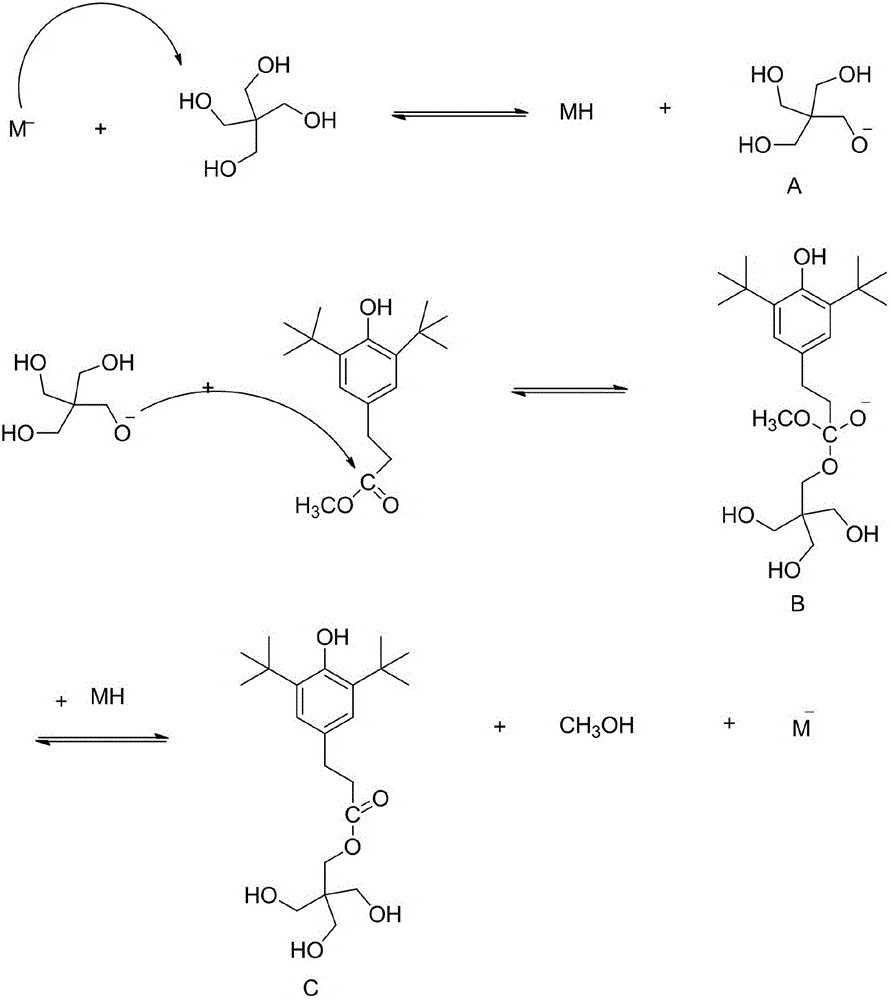
Method for preparing hindered phenol antioxygen 1010
A technology for hindered phenol antioxidant and organic solvent is applied in the field of preparing hindered phenol antioxidant 1010, which can solve the problems of low reaction rate, high metal content in the product, and difficulty in removing organic metals, and achieves high catalytic activity and no heavy metals. , the effect of not easy to color
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Take 4.63 g of n-chlorobutane and 6.85 g of N-methylimidazole in a four-necked flask, and react at 50°C for 12 hours under the protection of nitrogen. The mixed solvent of acetonitrile and ethyl acetate was recrystallized to obtain the ionic liquid [Bmim]Cl.
[0021] Dissolve 8.75 g of the above ionic liquid in 50 ml of dichloromethane, add 2.0 g of solid sodium hydroxide particles, stir at room temperature for 10 h, remove the precipitate by suction filtration, and remove the solvent by rotary evaporation to obtain the crude ionic liquid [Bmim]OH. The crude product was washed 3 times with anhydrous ether, evaporated under reduced pressure to remove the ether, and dried in vacuum at 80°C to constant weight to obtain the basic ionic liquid [Bmim]OH. .
Embodiment 2
[0023] Take 0.05 mol of the ionic liquid intermediate [Bmim]Cl and dissolve it in 50 ml of isopropanol, add 2.5 times the amount of KOAc, stir at 25° C. for 10 h, and filter out the insoluble matter. The filtrate was evaporated to remove the solvent to obtain a light yellow oil. Dissolve the crude product in dichloromethane, add activated carbon powder to it and stir for 10 h, filter, remove the solvent by rotary evaporation of the filtrate, and dry in vacuo to obtain a colorless oil, which is the target product [Bmim]OAc.
Embodiment 3
[0025] Dissolve 0.05 mol of [Bmim]Cl in 50 ml of isopropanol, add 2 times the amount of sodium carbonate, stir and react at 60°C for 8 hours, filter, filter and rotary evaporate to obtain an oily liquid, and dry in vacuo to obtain the final product [Bmim] 2 CO 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com