Screening and application of plant antiviral new target PsbO1
An anti-virus, plant technology, applied in applications, plant peptides, plant products, etc., can solve problems such as loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Immunoprecipitation
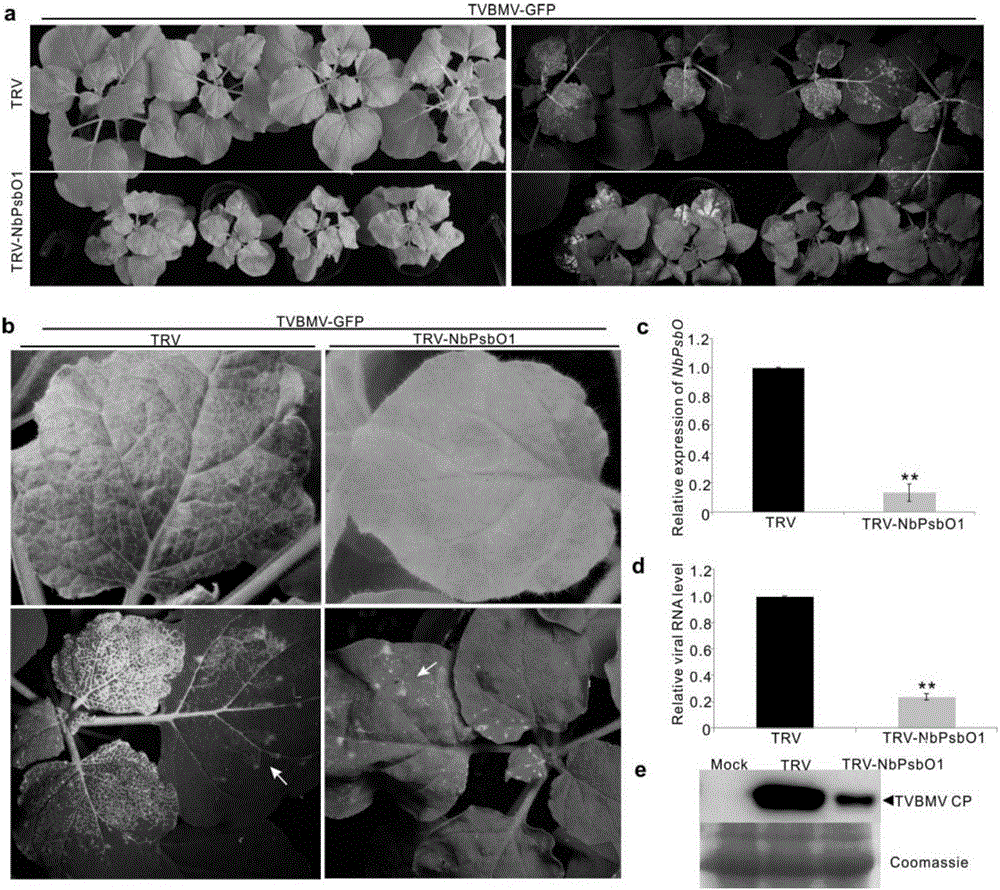
[0026] Take N. benthamiana leaves 7 days after infection with TVBMV-GFP or TVBMV-6K1GFP, add liquid nitrogen to a mortar, grind them thoroughly to powder, and add Extraction Buffer to extract total plant protein.
[0027] The composition of Extraction Buffer is as follows:
[0028] 50mM Tris-Cl, pH 7.5,
[0029] 150mM NaCl,
[0030] 10% [v / v] glycerol,
[0031] 0.5% [v / v] Nonidet P-40,
[0032] 1 tablet protease inhibitor cocktail
[0033] Centrifuge at 20,000 g for 10 min at 4°C, add the supernatant to GFP antibody-coupled agarose beads, incubate at 4°C for 3 hours, wash 5 times with Extraction Buffer without Nonidet P-40, add 2×SDS loading buffer, Cook in boiling water for 10 minutes, and separate protein bands by SDS-PAGE electrophoresis.
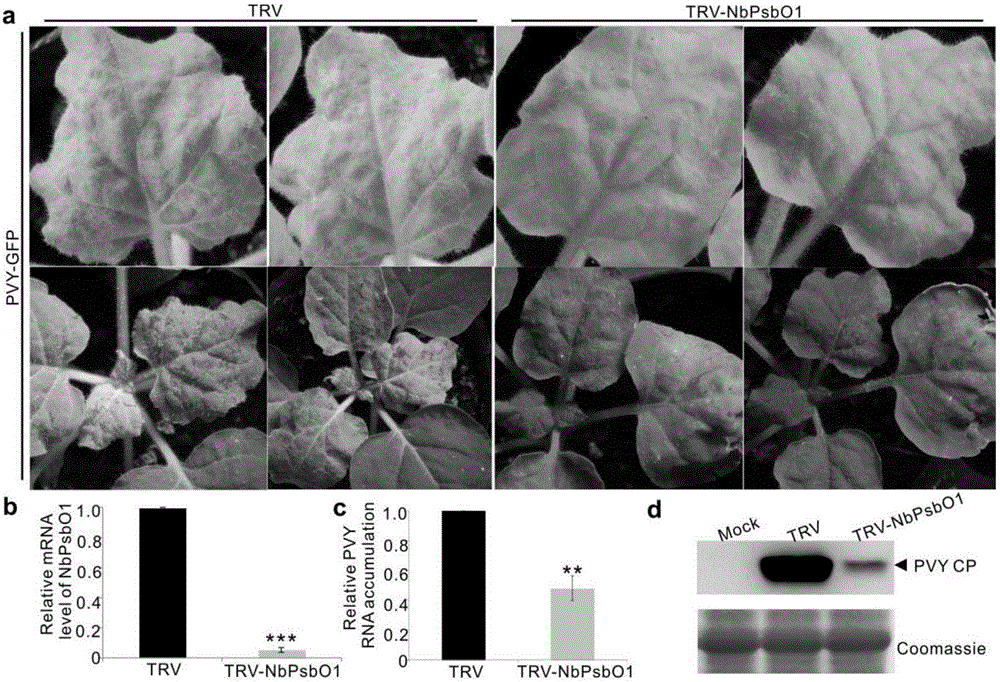
[0034] The protein band was excised and sent to Shanghai Jiyun Biotechnology Company for mass spectrometric identification. The results showed that there were 4 peptides homologous to PsbO1,...
Embodiment 2
[0035] Example 2: Gene Amplification
[0036] The PsbO1 gene was amplified by reverse transcription PCR using total plant RNA as a template. The reverse transcriptase used was M-MLV, and the DNA polymerase was Phusion high-fidelity polymerase.
[0037] The reverse transcription reaction system is as follows:
[0038]
[0039] The PCR reaction system is as follows:
[0040]
[0041] The obtained PsbO1 gene is 999bp in length, its sequence is shown in Seq ID No.7, and the encoded amino acid sequence is shown in Seq ID No.8.
Embodiment 3
[0042] Embodiment 3: Verification of the interaction between PsbO1 and TVBMV 6K1, 6K2
[0043] 6K1 and 6K2 can interact. PsbO1 in the 6K1GFP complex may directly interact with 6K1, or directly interact with 6K2, and indirectly interact with 6K1 through 6K2. PsbO1, TVBMV 6K1 and 6K2 genes were cloned into co-immunoprecipitation vectors pCam35S:HA and pCam35S:GFP, bimolecular fluorescent complementation vectors pYN and pYC, respectively.
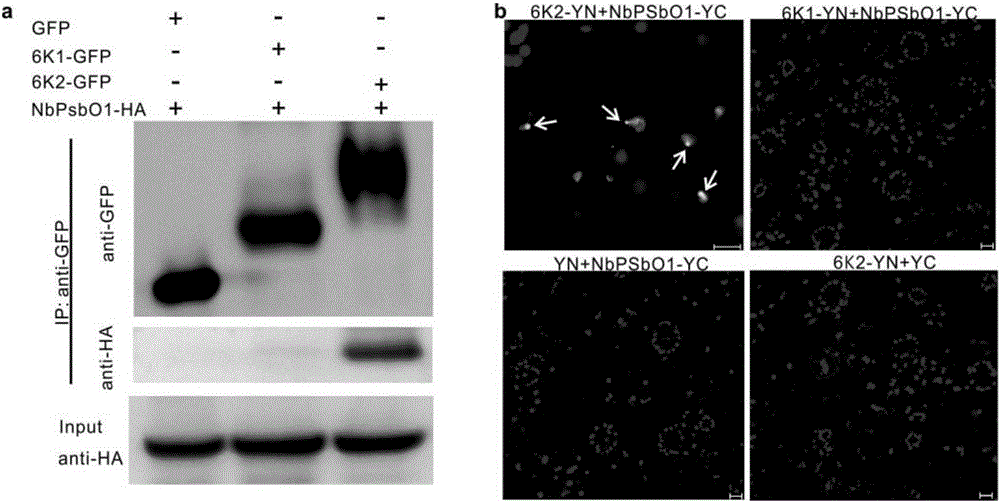
[0044] After the recombinant co-immunoprecipitation vector was transformed into Agrobacterium, the leaves of Nicotiana benthamiana were infiltrated. After 3 days, the total plant protein was extracted and added to GFP antibody-conjugated agarose beads for immunoprecipitation. Protein interaction was detected by Western blot using HA antibody. PsbO1 co-immunoprecipitated with 6K2 and co-immunoprecipitated with 6K1 ( figure 1 , a), indicating that PsbO1 can directly interact with 6K2, but not with 6K1.
[0045] After the recombined bimolecu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com