A novel organic ligand and its preparation method, novel ruthenium complexes and fluorescent probes
A technology of organic complexes and organic ligands, applied in ruthenium organic compounds, platinum group organic compounds, organic chemistry, etc., can solve the problems of unstable fluorescence emission lifetime, short water solubility, etc., and achieve excellent optical properties and excited state lifetime Long-lasting, biocompatible effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] The preparation method of fpic is as follows: add 5-fluoroindolecarboxylic acid to benzene or pyridine, slowly add thionyl chloride dropwise, heat to reflux, concentrate until crystals are precipitated, and then add 5-amino-1,10-phenanthroxine morphine, stirred, and the precipitate was collected and dried. Its chemical reaction formula is:
[0047]
[0048] The ligand fpic, the organic complex of ruthenium, and the reaction solvent ethanol were mixed, refluxed for 6h under nitrogen, cooled to room temperature, filtered, and NaClO was added to the filtrate 4 Aqueous solution, precipitated, suction filtered, washed, dried to obtain the ruthenium complex.
[0049]When the organic complex of ruthenium is bis(1,10-phenanthroline)-dichloro-ruthenium dihydrate, the molecular structure of the novel ruthenium complex is as shown in formula (a):
[0050]
[0051] The organic complex of ruthenium is bis(2,2'-bipyridine)-dichloro-ruthenium dihydrate, and the molecular struc...
Embodiment 1
[0055] The synthesis of embodiment 1 organic ligand fpic
[0056] 5-fluoroindole-2-carboxylic acid (1.27g, 9.0mmol) was added to benzene, heated to reflux, slowly added thionyl chloride dropwise, and the suspension was heated to reflux for min; then, the mixture was concentrated in vacuo until light Yellow-green crystals precipitated to give 5-fluoroindole-2-yl chloride. A benzene solution of 5-fluoroindole-2-acyl chloride was added dropwise to a solution of 5-amino-1,10-phenanthroline (1.9 g, 9.0 mmol) in pyridine, and the resulting mixture was stirred at room temperature Overnight; let the precipitate stand, filter and collect the precipitate, and then crystallize in ethanol solution to obtain a khaki solid with a yield of 2.918 g and a yield of 91%.
[0057] Fpic- 1 H NMR(400MHz,DMSO,ppm):δ12.04(3H,s),10.96(3H,s),9.25(4H,d,J=3.3Hz),9.22-9.14(3H,m),8.97-8.72 (7H,m),8.35(3H,s),8.16-7.95(7H,m),7.62(3H,d,J=1.5Hz),7.57-7.40(8H,m),7.26(1H,d,J =17.4Hz),7.20-6.99(4H,m),5.37-2.0...
Embodiment 2
[0058] Example 2 Complex Ru[(phen) 2 (fpic)] (ClO 4 ) 2 2H 2 Preparation of O
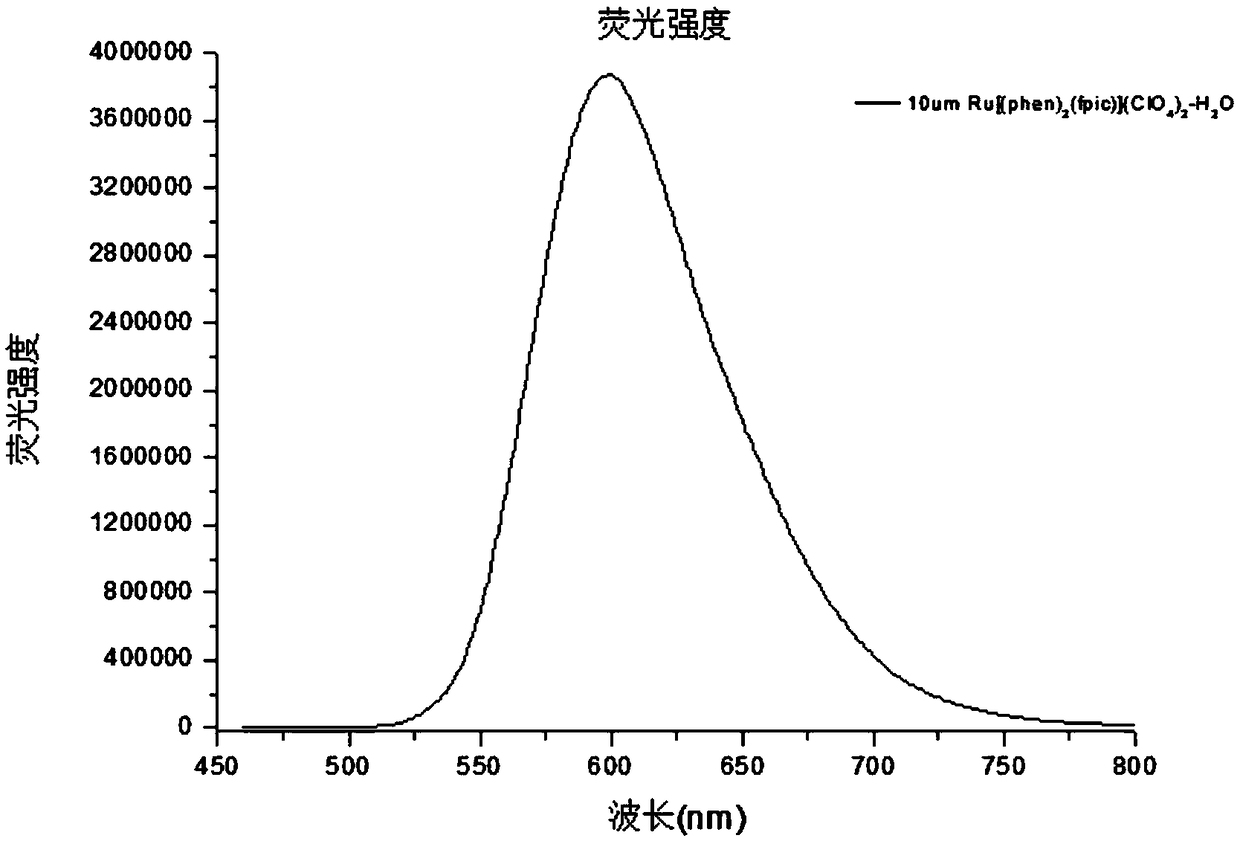
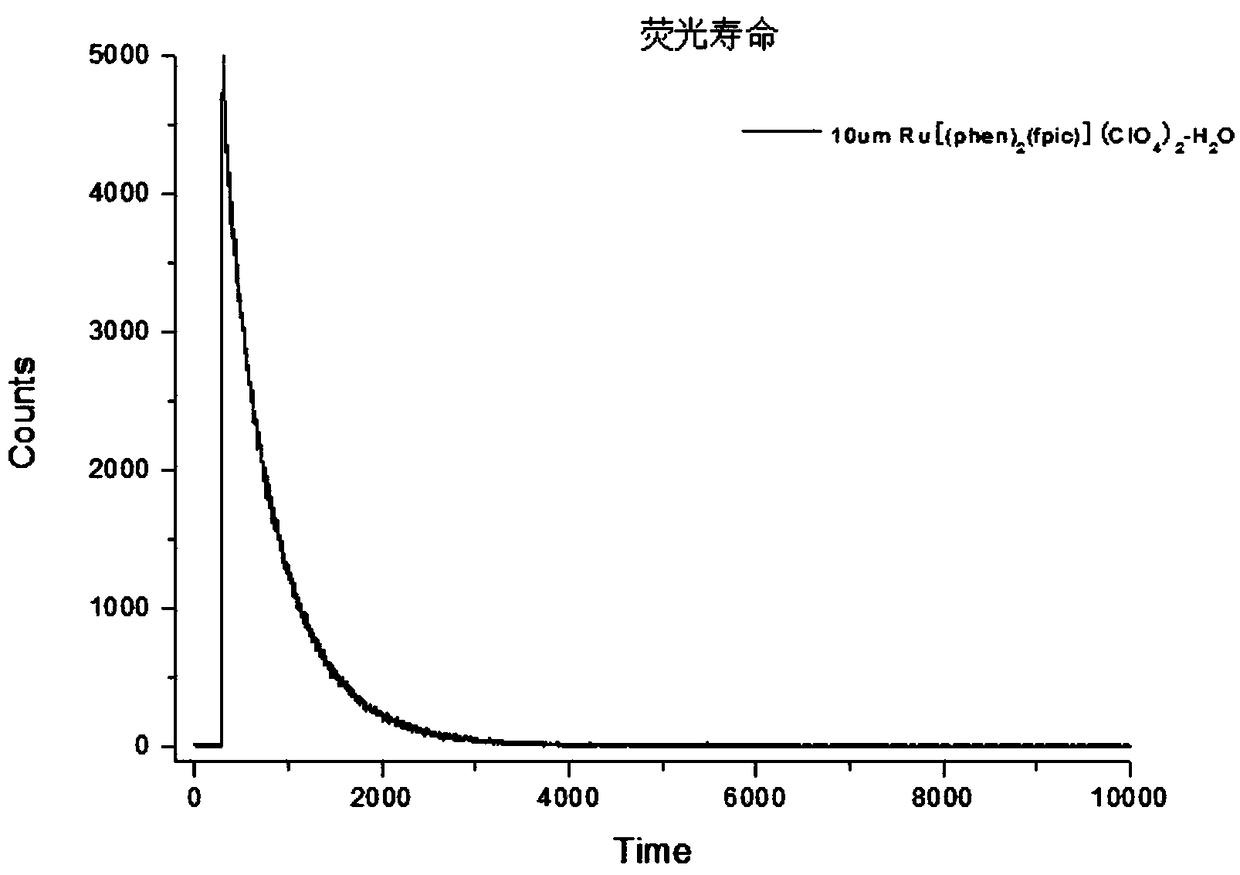
[0059] 0.568g (1mmo1) of Ru(phen) 2 Cl 2 2H 2 O. 0.356g (1mmol) of fpic was added to a three-necked flask, and then ethanol was added, and the resulting mixture was refluxed for 6h under nitrogen, cooled to room temperature, and filtered. Add NaClO to the filtrate 4 , stand still, filter with suction, wash the precipitate with water and diethyl ether for several times, and then dry it in vacuum to obtain an orange-red solid product with a yield of 2.918 g and a yield of 75%.
[0060] Ru[(phen) 2 (fpic)] - 1 H NMR (400MHz, DMSO, ppm): δ11.98 (22H, d, J = 18.2Hz), 10.95 (20H, s), 8.93-8.73 (129H, m), 8.62 (23H, s), 8.40 (84H ,s),8.23-8.00(126H,m),7.99-7.70(130H,m),7.67-7.38(79H,m),7.28(7H,s),7.13(28H,dtd,J=24.7,9.2, 2.5Hz), 3.35(629H, s), 2.47(303H, d, J=29.2Hz), 2.36-1.23(33H, m), 1.61(21H, d, J=304.8Hz), 1.23(11H, s) , 1.00 (9H,d,J=118.3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com