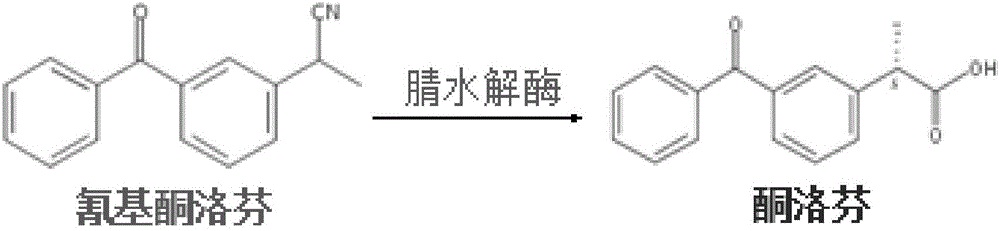
Nitrilase mutant as well as coding gene and application thereof
A nitrilase and mutant technology, applied in the fields of hydrolase, application, genetic engineering, etc., can solve the problems that levoketoprofen has no pharmacological activity and ketoprofen lacks chiral selectivity, etc., and achieves high enzyme activity and/or Or stereoselectivity, reduce production cost, improve production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
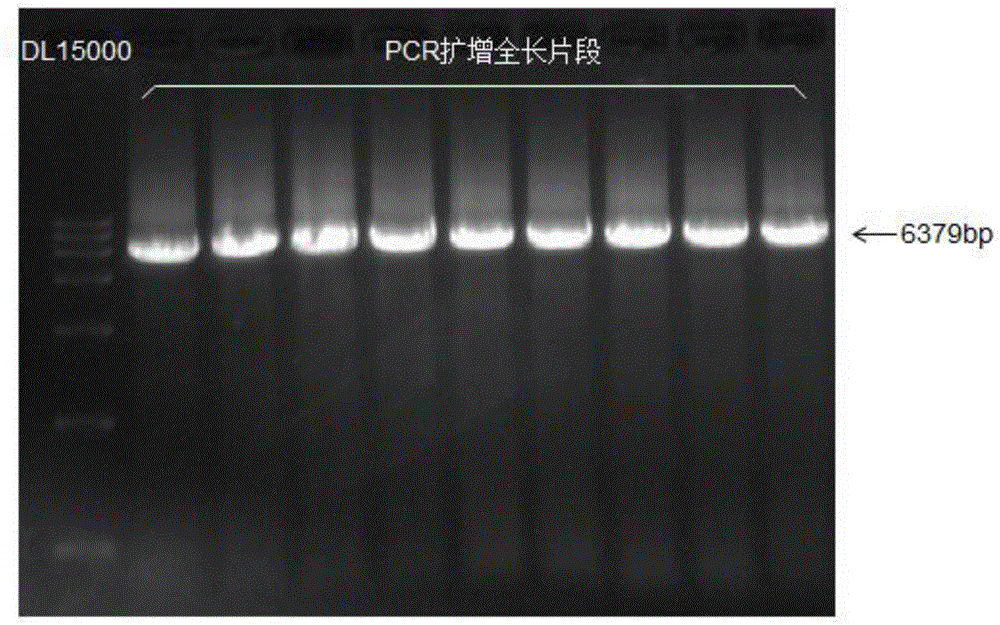
[0070] Cloning of embodiment 1 wild-type nitrilase gene
[0071] (1) Acquisition of wild-type nitrilase gene
[0072] A strain of Alcaligenes faecalis HEC-AF001 was screened from the soil near industrial wastewater. The total genomic DNA was extracted using the OMEGA bacterial genome kit, and a gene fragment of about 1.1 kb in length was amplified using the following primers.
[0073] HEC-AF001-F: CCCATATGCAGACAAGAAAAATC (SEQ ID NO: 17)
[0074] HEC-AF001-R: CCAAGCTTTCAGGACGGTTCTTG (SEQ ID NO: 18)
[0075] The PCR reaction system is shown in Table 1
[0076] Table 1
[0077] Element System (μl) 5×PrimeSTAR PCR HS Buffer 10 Primer 1 1 Primer 2 1 Template (<0.2μg)
1 dNTPs (2.5mM) 4 PrimeSTAR PCR HS Polymerase 0.5 wxya 2 o
50
[0078] PCR amplification program: 98°C, pre-denaturation for 20s; 98°C, denaturation for 10s; 60°C for 10s; 72°C for 60s; repeat 30 cycles; 72°C for 10 minutes.
[0079] The PCR product w...
Embodiment 2
[0081] Embodiment 2 Preparation of recombinant expression plasmid and recombinant expression transformant
[0082] The PCR product obtained in Example 1 was double-digested with restriction endonucleases NdeI and HindIII at 37°C for 4 h, purified by agarose gel electrophoresis, and the target fragment was recovered using an agarose gel DNA recovery kit, which contained the correct Insert snippet. The target fragment was mixed with the plasmid pET28a digested with NdeI and HindIII, and ligated at 16°C for 4 hours under the action of T4 DNA ligase to obtain the recombinant expression plasmid pET28a-NIT1.
[0083]The above recombinant expression plasmids were transformed into E.coli Top10 competent cells. On the resistant plate containing kanamycin (medium composition LB medium, peptone 10g / L, yeast extract 5g / L, sodium chloride 10g / L and agar 2%, antibiotic content 50mg / L) Screening was carried out, single clones were picked, and recombinant strains were cultivated. After plas...
Embodiment 3
[0084] Expression of embodiment 3 recombinant nitrilase
[0085] The recombinant Escherichia coli obtained in Example 2 was inoculated into LB medium containing kanamycin (50 mg / L), and cultured with shaking at 37° C. overnight. According to the inoculum amount of 2‰ (v / v), insert into the 250ml Erlenmeyer flask that 50ml LB culture medium is housed, put 37 ℃, 180rpm shaker shaking culture. When the OD of the culture medium 600 When it reaches 0.8, add IPTG with a final concentration of 0.5mmol / L for induction. After induction at 30°C for 8 hours, the culture medium was centrifuged to collect the cells to obtain 0.3 g of wet cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com