Thermophilic esterase derived from aquifex aeolicus strain and functional verification of thermophilic esterase
A thermophilic ester and esterase technology, applied in the field of bioengineering, can solve problems such as difficult acquisition and slow growth of thermophilic bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Obtaining and Gene Sequence Analysis of A.aeolicus Recombinant Thermophilic Esterase Gene
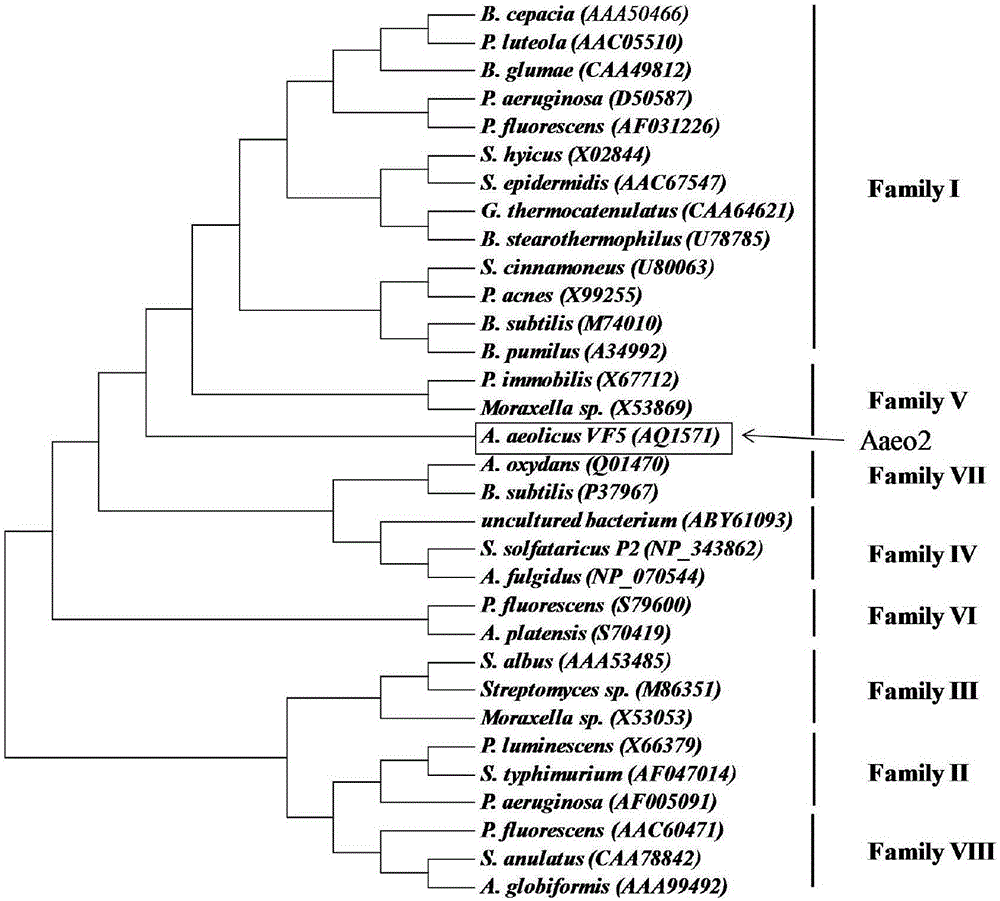
[0060] According to the characteristics of the conserved sequence "Gly-Xaa-Ser-Xaa-Gly" in the primary structure of esterase and the key enzyme active site "Ser, His, Asp", two hypothetical protein sequences were selected from the genome of the A.aeolicus strain, Blast analysis was performed on it in the SWISS-PROT database, and the Aaeo1 sequence was found to have 27% similarity with a methylester esterase (Reference sequence: Q82SL8.2) derived from Nitrosomonas europaea. The sequence of Aaeo2 has 25% similarity with a mycophenolic acid acyl-glucuronide esterase (Reference sequence: Q5E9H9.1) derived from Bos taurus. Therefore, it can be preliminarily guessed that these two sequences may encode esterase proteins.
[0061] Five thermophilic esterases / lipases that have been studied were selected and compared with two thermophilic esterases of A. aeolicus for sequence c...
Embodiment 2
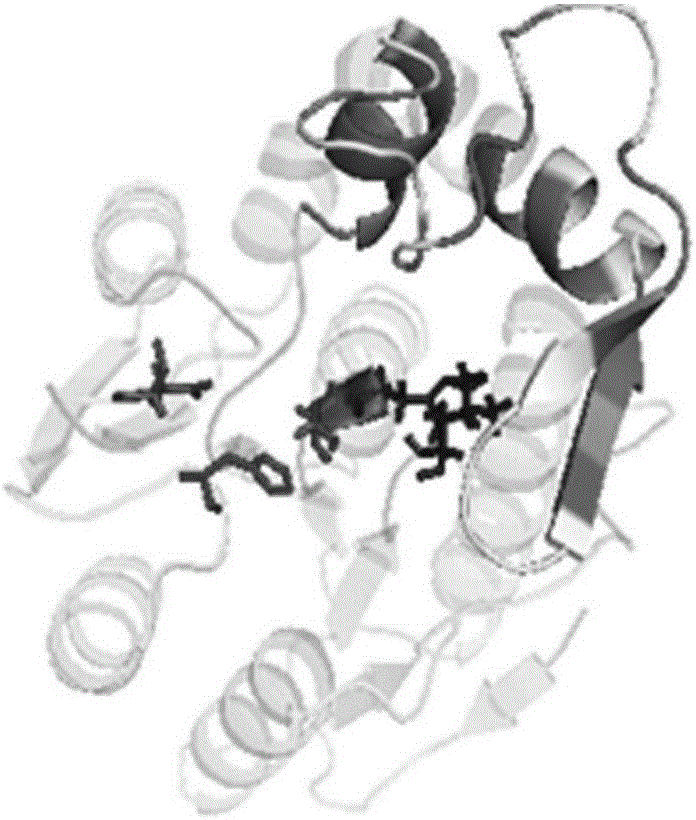
[0065] Example 2 Obtaining the three-dimensional predicted structures of two thermophilic esterases from A. aeolicus by using the "homology modeling" method
[0066] A thermophilic esterase gene Aaeo1 derived from A. aeolicus is 621bp in length, encodes 207 amino acids, and has a molecular weight of 23.4kDa. The nucleic acid sequence and protein sequence of the protein are shown in SEQ ID NO:3 and SEQ ID NO:1. Another thermophilic esterase gene Aaeo2 is 678bp in length, encodes 226 amino acids, and has a molecular weight of 26.8kDa. The nucleic acid sequence and protein sequence of the protein are shown in SEQ ID: 4 and SEQ ID NO: 2.
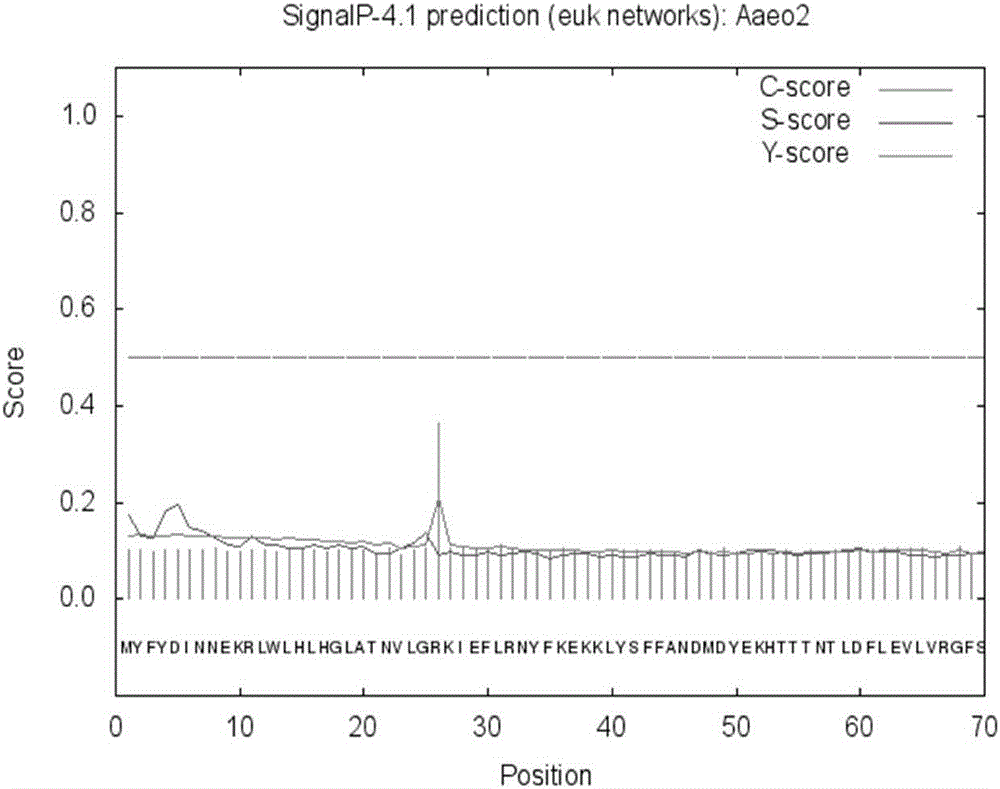
[0067] According to the SignalP prediction, the N-terminus of the hypothetical protein Aaeo2 has no possibility of signal peptide (see figure 2 ).
[0068] Submit the amino acid sequences of the two thermophilic esterases of A.aeolicus to the I-TASSER protein online modeling server (http: / / zhanglab.ccmb.med.umich.edu / I-TASSER / ) for homology mo...
Embodiment 3
[0069] Example 3 Construction of eukaryotic expression vectors for two kinds of aeolicus thermophilic esterases, recombinant expression and protein expression thereof
[0070] 1. Construction of eukaryotic expression vector
[0071] According to the codon preference of Pichia pastoris, the two thermophilic esterase genes of A. aeolicus introduced 6xHis tag at the C-terminus of the DNA coding frame, and introduced AvrII and NotI restriction enzyme sites on the 5' and 3' sides, respectively , the optimized sequences, namely SEQ ID NO: 5 (Aaeo1) and SEQ ID NO: 6 (Aaeo2), were synthesized by the biological company, and connected to the expression vector pPIC9K to obtain the recombinant expression vectors pPIC9K-Aaeo1 and pPIC9K-Aaeo2.
[0072] The recombinant expression vector plasmid pPIC9K-Aaeo2 was digested with AvrII and NotI, and digested at 37°C for 30min. Double enzyme digestion for simple identification, 1% agarose nucleic acid gel such as Figure 4 As shown, the positive ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com