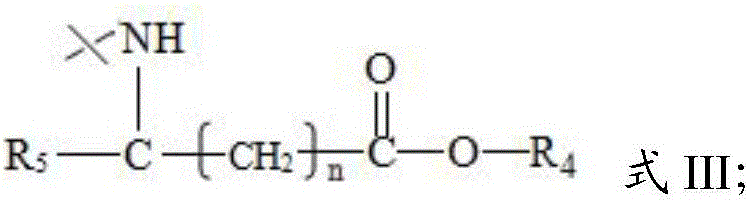Peotopanaxadiol ginsenoside derivative and preparation method and application thereof
A ginsenoside and diol type technology, applied in the field of medicine, can solve the problems of low activity, lack of practical application value, cytotoxicity, etc., and achieve the effects of low cytotoxicity, lowering the level of low-density lipoprotein cholesterol, and good anti-inflammatory effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048]The present invention provides the preparation method of diol type ginsenoside derivative described in above-mentioned technical scheme, comprises the following steps:
[0049] (1) Carrying out a nucleophilic substitution reaction between the parent compound and the acid anhydride in the presence of an alkaline reagent to obtain the first intermediate product;
[0050] (2) performing an oxidation reaction on the first intermediate product in the step (1) in the presence of an oxidizing agent and an organic solvent to obtain a second intermediate product;
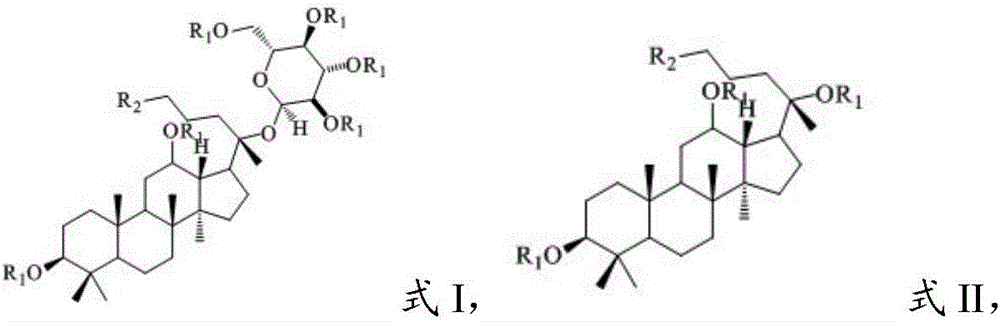
[0051] (3) Reductive amination reaction of the second intermediate product and the amino compound in the presence of an organic solvent and a reducing agent in the step (2) to obtain a diol-type ginsenoside having a structure shown in formula I or formula II derivative;
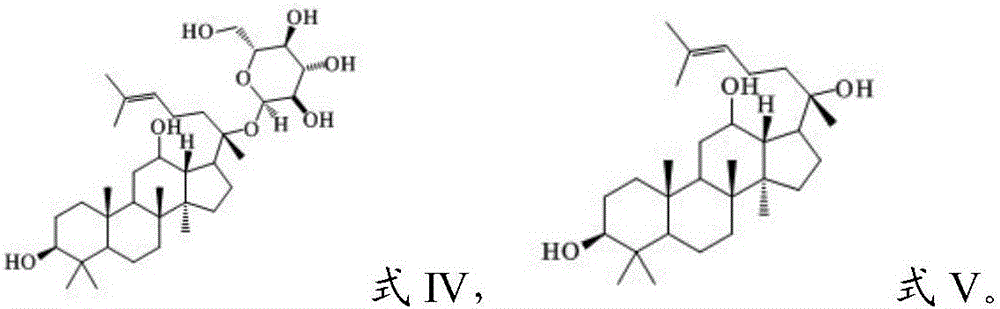
[0052] Wherein, in the step (1), the parent compound has the structure shown in formula IV or V:
[0053]
[0054] In the present invention, the...
Embodiment 1
[0098] Mix 1g (16mmol) of the compound having the structure shown in formula III with 10mL of acetic anhydride and 10mL of pyridine, and perform a nucleophilic substitution reaction at 40°C and 900rpm magnetic stirring for 3h; distill the obtained crude product with 6mL Ethyl acetate was mixed, and the silica gel was used for column chromatography after mixing the sample. The mixture of ethyl acetate and n-hexane was used as the eluent (the volume ratio of ethyl acetate and n-hexane was 1:5), and 1.3 g of white solid was obtained. Compound 1, yield 90%. The structure of the compound 1 is:
[0099]
Embodiment 2
[0101] Mix 1g (11mmol) of the compound 1 prepared in Example 1, 5mL of dichloromethane, and 0.2g (11mmol) of m-chloroperoxybenzoic acid, and carry out the primary oxidation reaction at room temperature for 1h; Add 25mL of water to the material, stir it magnetically at 1100rpm for 1h, a white solid precipitates out, filter to obtain a clear solution, distill off the solvent under reduced pressure, mix the obtained crude product with 8mL of ethyl acetate, mix the sample with silica gel, and use it for column chromatography. Using a mixture of ethyl acetate and n-hexane as the eluent (the volume ratio of ethyl acetate and n-hexane is 1:8), 0.87 g of compound 2 was obtained as a white solid with a yield of 90%. The structure of the compound 2 is:
[0102]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com