Preparation method of (1,5-cyclooctadiene) rhodium chloride (I) dimers
A technology of cyclooctadiene and rhodium chloride is applied in the field of preparation of rhodium chloride dimer, and can solve the problems of easy oxidation of products, prolonged reaction time, and inability to stand for a long time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
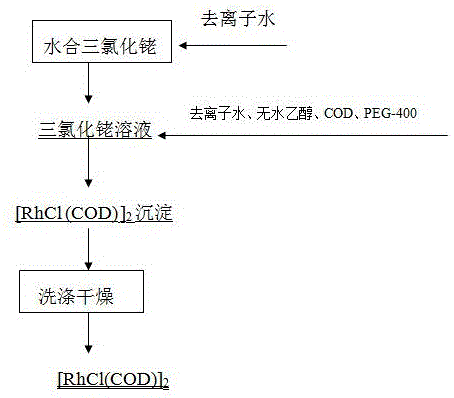
[0019] Weigh 20g of hydrated rhodium trichloride (Rh: 39.5%), dissolve it with 240ml of deionized water (3%), and add it to a 1L reactor. The top of the reactor is connected to a condenser, and 240ml of absolute ethanol is added. The temperature of the oil bath rose to 35°C, and 90ml of a mixed solution of 1,5-cyclooctadiene COD and anhydrous ethanol was slowly added dropwise. The volume ratio of 1,5-cyclooctadiene COD to anhydrous ethanol was 0.5: The molar ratio of 1,1,5-cyclooctadiene COD to rhodium is 2.6:1. After the mixed solution of 1,5-cyclooctadiene COD and absolute ethanol is dripped, add 20ml of phase transfer catalyst PEG-400 , Keep the temperature of the oil bath at 35°C for 7 hours, and precipitate an orange precipitate, cool to room temperature, filter with suction, wash with absolute ethanol and deionized water 3 times, dry and weigh. The product yield was 80.1%, and the elemental analysis results were C: 38.87%, H: 4.854%, and Rh: 41.54%.
Embodiment 2
[0021] Weigh 20g of hydrated rhodium trichloride (Rh: 39.5%), dissolve it with 79ml of deionized water (8%), and add it to a 1L reactor. The top of the reactor is connected to a condenser. Add 237ml of absolute ethanol, stir and heat. The temperature of the oil bath rose to 35°C, and 110ml of a mixed solution of 1,5-cyclooctadiene COD and anhydrous ethanol was slowly added dropwise. The volume ratio of 1,5-cyclooctadiene COD to anhydrous ethanol was 1.2: The molar ratio of 1,1,5-cyclooctadiene COD to rhodium is 5.1:1. When the mixed solution of 1,5-cyclooctadiene COD and absolute ethanol is dripped, add 60ml phase transfer catalyst PEG-400 , Keep the oil bath temperature at 80℃ and react for 4h, and the orange-yellow precipitate is precipitated, cooled to room temperature, suction filtered, washed with absolute ethanol and deionized water for 3 times, and dried and weighed. The product yield was 82.2%, and the elemental analysis results were C: 38.76%, H: 4.860%, and Rh: 41.57%...
Embodiment 3
[0023] Weigh 20g of hydrated rhodium trichloride, dissolve it with 120ml of deionized water (5.6%), and add it to a 1L reactor. The reactor is connected with a condenser, and the reactor is connected with a condenser. Add 240ml of absolute ethanol, stir and heat , When the temperature of the oil bath rises to 35℃, start to slowly add 80ml of a mixed solution of 1,5-cyclooctadiene COD and anhydrous ethanol, in which the volume ratio of 1,5-cyclooctadiene COD to anhydrous ethanol is 1.0:1, The molar ratio of 1,5-cyclooctadiene COD to rhodium is 3.4:1. When the mixed solution of 1,5-cyclooctadiene COD and absolute ethanol is added dropwise, add 40ml phase transfer catalyst PEG -400, keep the oil bath temperature at 40℃ and react for 5 hours, and orange-yellow precipitate is precipitated, cooled to room temperature, suction filtered, washed with absolute ethanol and deionized water for 3 times, and dried and weighed. The product yield was 82.1%, and the elemental analysis results w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com