Novel method for preparation of vortioxetine hydrobromide crystal form alpha
A technology of vortioxetine hydrobromide and crystal form, which is applied in the fields of pharmacy and chemical industry, and the new preparation field of vortioxetine hydrobromide crystal form α, which can solve the problem of low sample purity and unsatisfactory performance. To the problems of pharmaceutical grade requirements and complex operation, it can achieve the effects of good preparation processability, excellent stability, and excellent preparation dissolution rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
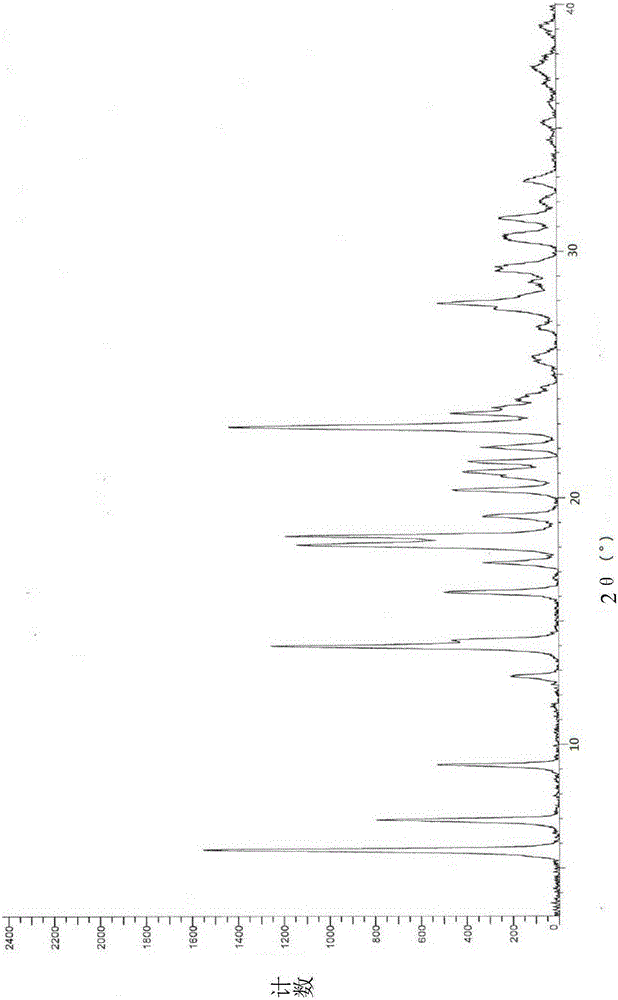
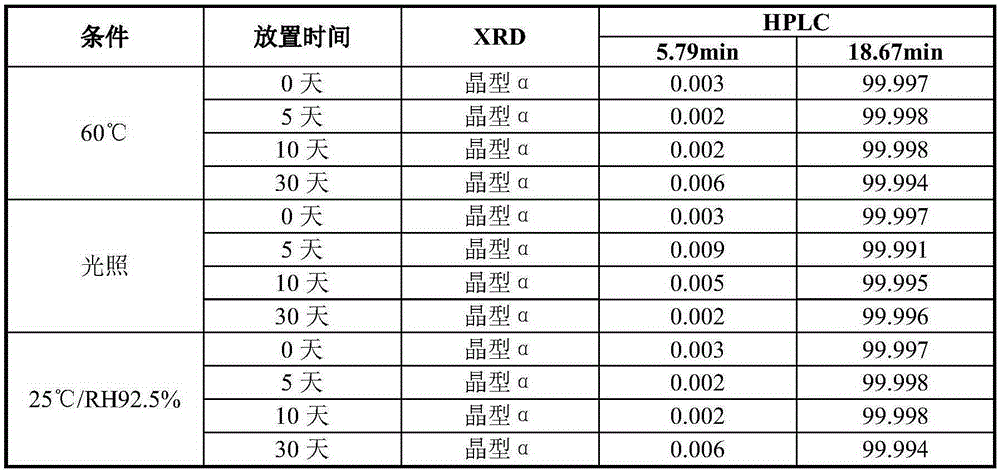
[0021] Dissolve 1 g of vortioxetine hydrobromide in 10 ml of N,N-dimethylformamide to form a solution, and add the resulting solution dropwise to 100 ml of methyl tert-butyl ether at a temperature of 20°C, stirring After 10 minutes, it was filtered and dried to obtain 0.78g of crystal form α, which was detected by X-ray diffraction, and the results were as follows: figure 1 As shown, it is basically consistent with the XRD spectrum of crystal form α reported in patent CN101472906B.
Embodiment 2
[0023] Dissolve 1 g of vortioxetine hydrobromide in 10 ml of N,N-dimethylacetamide, heat to 40°C to form a solution, and add the resulting solution dropwise to 150 ml of methyl tert-butyl In base ether, after stirring for 10 minutes, filter and dry to obtain 0.82g crystal form α, and its XRD pattern is consistent with figure 1 Basically the same.
Embodiment 3
[0025] Dissolve 2 g of vortioxetine hydrobromide in 10 ml of chloroform to form a solution. Add the resulting solution dropwise to 100 ml of isopropyl ether at a temperature of -15° C., keep stirring for 10 minutes, filter and dry to obtain 1.60 g crystal form α, its XRD pattern and figure 1 Basically the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com