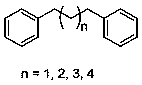
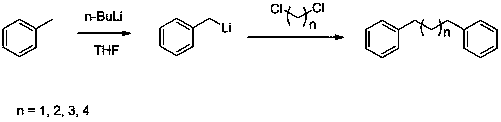
A kind of method of synthesizing diphenyl alkane compound
A technology for diphenylalkanes and compounds, applied in the field of organic synthesis, can solve the problems of long route, high substrate cost, limited application and the like, and achieves the effects of optimizing the reaction process, low production cost and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0013] Example 1 Synthesis of 1,3-diphenylpropane
[0014] Under argon protection, 10 mL of toluene was added to the dry reaction vessel at 20 °C, then 8 mL of n-butyllithium (2.5 M n-hexane solution) was added dropwise with stirring, and 10 mL of tetrahydrofuran was slowly added dropwise at the end of the addition. After reacting at 20 ºC for 3 hours, add 0.85 g of dichloromethane dropwise to the reaction system, and react for 3 hours after dropping, add water to quench the reaction, then separate the layers, dry the organic layer with anhydrous magnesium sulfate, filter and distill under reduced pressure to obtain 1,3-diphenylpropane 1.24g, yield 63%. 1 HNMR (400MHz, CDCl 3 ) δ = 1.96-2.06 (m, 2H), 2.66-2.74 (m, 4H), 7.19-7.25 (m, 6H), 7.27-7.35 (m, 4H).
example 2
[0015] Example 2 Synthesis of 1,4-diphenylbutane
[0016] Under argon protection, 10 mL of toluene was added to the dry reaction vessel at 20 °C, then 8 mL of n-butyllithium (2.5 M n-hexane solution) was added dropwise with stirring, and 10 mL of tetrahydrofuran was slowly added dropwise at the end of the addition. After reacting at 20 ºC for 3 hours, add 1.0 g of 1,2-dichloroethane dropwise to the reaction system, and react for 3 hours after dropping, add water to quench the reaction, then separate the layers, dry the organic layer with anhydrous magnesium sulfate, and filter After vacuum distillation, 1.43 g of 1,4-diphenylbutane was obtained with a yield of 68%. 1 HNMR (400 MHz, CDCl 3 ) δ =1.65-1.73 (m, 4H), 2.61-2.68(m, 4H),7.14-7.22 (m, 6H),7.25-7.32 (m, 4H).
example 3
[0017] Example 3 Synthesis of 1,5-diphenylpentane
[0018] Under argon protection, 5 mL of toluene was added to the dry reaction vessel at 10 °C, then 8 mL of n-butyllithium (2.5 M n-hexane solution) was added dropwise with stirring, and 5 mL of tetrahydrofuran was slowly added dropwise at the end of the addition. After reacting at 10 ºC for 5 hours, add 1.13 g of 1,3-dichloropropane dropwise to the reaction system, and react for 3 hours after dropping, add water to quench the reaction, then separate the layers, dry the organic layer with anhydrous magnesium sulfate, and filter Distilled under reduced pressure to obtain 2.0 g of 1,5-diphenylpentane with a yield of 89%. 1 HNMR (400 MHz, CDCl 3 ) δ = 1.96-2.06 (m, 2H), 2.66-2.74 (m, 4H), 7.19-7.25 (m, 6H),7.27-7.35 (m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com