Method for determining content of beta-hydroxy-beta-methylbutyric acid in soybean peptide protein powder
A technology of methyl butyric acid and protein powder, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems that have not been reported before, and achieve the effects of economical effectiveness, good reproducibility, and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Preparation of sample solution
[0023] Preparation of the sample solution: take 0.6g of the sample, accurately weigh it, put it in a 25ml measuring bottle, add 5ml of 0.1mol / L hydrochloric acid solution, shake vigorously to dissolve the solid, dilute to the mark with acetonitrile, shake for 1 minute, filter , Precisely draw 2.0ml of the filtrate, put it in a 5ml measuring bottle, dilute to the mark with 0.1mol / L hydrochloric acid solution, shake well, filter through a 0.45μm filter membrane, take the filtrate as the sample solution;
[0024] Preparation of negative solution: Take 0.6g of negative sample, weigh it accurately, put it in a 25ml measuring bottle, add 5ml of 0.1mol / L hydrochloric acid solution, shake vigorously to dissolve the solid, dilute to the mark with acetonitrile, shake for 1 minute, filter After that, accurately draw 2.0ml of the filtrate, put it in a 5ml measuring bottle, dilute to the mark with 0.1mol / L hydrochloric acid solution, shake well, ...
Embodiment 2
[0054] Embodiment 2: Optimization of sample dissolution process
[0055] Take 2.3g of the sample, put it in a 100ml measuring bottle, and directly dissolve the sample with 0.1mol / L hydrochloric acid solution. Remove protein from sample solution to eliminate interference.
[0056] Take 0.6g each of the sample and the negative sample, put them in a 25ml measuring bottle, add 5ml of 0.1mol / L hydrochloric acid solution, shake vigorously to dissolve the solid, dilute to the mark with acetonitrile, shake for 1 minute, filter, and accurately absorb the filtrate Put 2.0ml in a 5ml measuring bottle, dilute to the mark with 0.1mol / L hydrochloric acid solution, shake well, filter through a 0.45μm filter membrane, and take the subsequent filtrate as the sample solution. Organic solvents can reduce the electrolytic constant of the solution, increase the attraction of different charges on protein molecules, and reduce the solubility of proteins and precipitate them; therefore, acetonitrile...
Embodiment 3
[0057] Embodiment 3: the selection of mobile phase
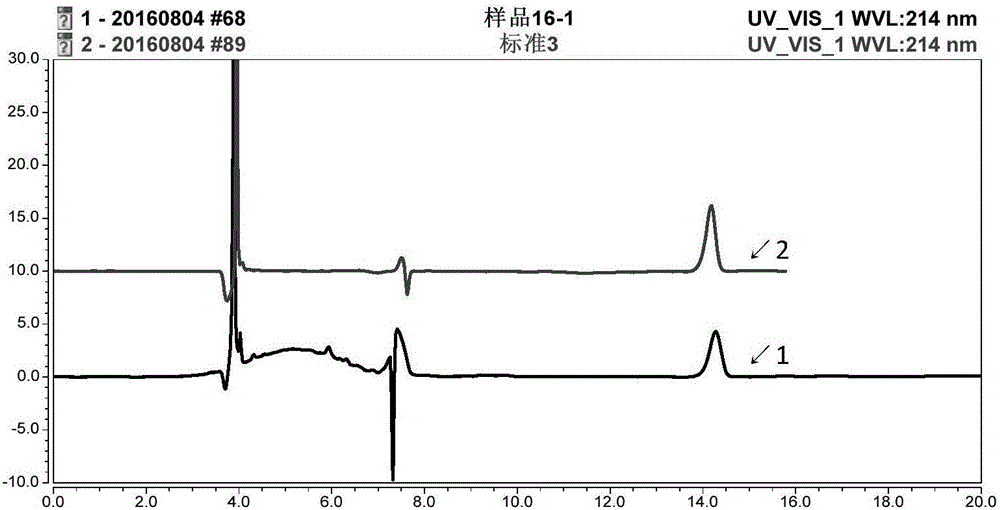
[0058] (1) Prepare sample solution and negative sample solution as described in "Example 1";
[0059] (2) Preparation of the control solution: Weigh about 60 mg of the β-hydroxy-β-methylbutyrate calcium hydrate standard substance, put it in a 50ml measuring bottle, dissolve it with 0.1mol / L hydrochloric acid solution and dilute to the mark to obtain the control product solution.
[0060] (3) Chromatographic conditions:
[0061] Column: Phenomex Uranus C 18 Chromatography column, 250mm×4.6mm, 5μm.
[0062] Flow rate: 0.5ml / min; Column temperature: 35°C; Injection volume: 5μl;
[0063] Mobile phase 1: 0.01mol / L sodium heptanesulfonate solution-acetonitrile (95:5) (10% phosphoric acid solution to adjust the pH value to 3.0);
[0064] Mobile phase 2: 0.01mol / L potassium dihydrogen phosphate solution-acetonitrile (95:5) (10% phosphoric acid solution to adjust the pH value to 2.6);
[0065] Test with mobile phase 1 and mobil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com