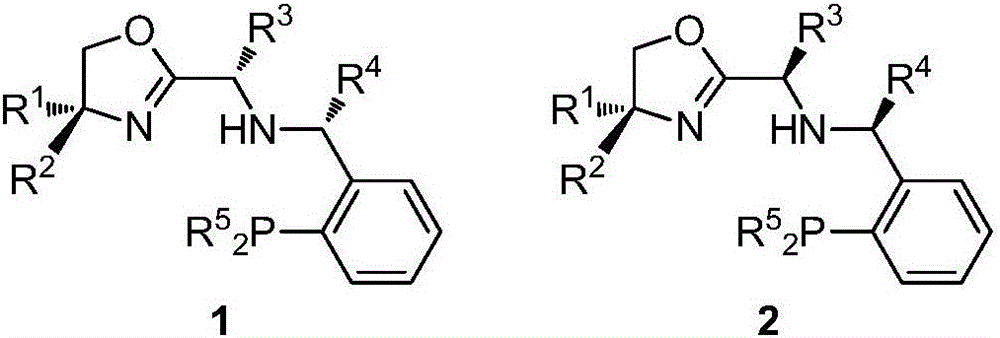
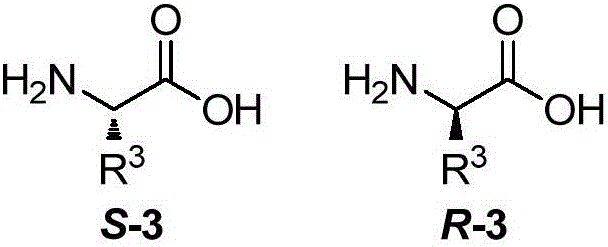Chiral oxazoline NNP type ligands as well as synthesis method and application thereof
A chiral oxazoline and ligand technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, carbon-based compound preparation, etc., can solve problems such as limiting the effect of catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] A kind of chiral oxazoline class NNP type ligand synthetic method is as follows:
[0092] (1) Synthesis of intermediate S-4a.
[0093]
[0094] Take a 500mL reaction flask, add chiral amino acid S-3a (4.5g, 38.8mmol), dioxane (40mL) and 10% sodium carbonate (100mL) to the reaction flask respectively, and place the reaction flask in an ice bath , stirred mechanically, added 9-fluorenylmethyl chloroformate (10.0 g, 38.8 mmol) and dioxane (100 mL) into the dropping funnel, slowly dropped into the reaction flask, gradually returned to room temperature and stirred overnight. After the reaction was completed, add 100 mL of water, extract three times with 50 mL of ether, take the water phase and put it in an ice bath to cool, and add 1M dilute HCl until the pH is 1. The aqueous solution was extracted three times with 50 mL of ethyl acetate. The oil phases were combined, dried with magnesium sulfate, filtered and spin-dried to obtain intermediate S-4a (12.6 g, 96%).
[00...
Embodiment 2
[0111] Example 2: The intermediate S-8a was prepared by the same method as in Example 1, and then the catalyst 1b was obtained by the following method.
[0112]
[0113] Take a 25mL dry reaction flask and fill it with argon gas to protect it. Add intermediate S-8a crude product (1.9g, 3.8mmol) and anhydrous dichloromethane (20mL) to the reaction flask respectively, cool to -70°C, and slowly add 3M benzene Magnesium bromide (5.1 mL, 15.2 mmol). After the reaction is completed, add saturated ammonium chloride solution to quench, add dichloromethane to extract and separate the liquids, dry the oil phase over magnesium sulfate, filter and spin dry. The catalyst 1b (511mg, 24%) was obtained by using ethyl acetate:petroleum ether=1:20 to pass through the column.
[0114] White solid.IR(KBr)v max 3427,2964,1655,1454,1434,1089,744,697cm -1 ; 1 H NMR (400MHz, CDCl 3 )δ8.01-7.98(m,1H),7.41-7.12(m,18H),7.04-6.92(m,6H),6.10(d,J=8.8Hz,1H),5.24(t,J=9.6Hz ,1H),4.55(t,J=9.2Hz,1H),4....
Embodiment 3
[0115] Example 3: The intermediate S-8a was prepared by the same method as in Example 1, and then the catalyst 1c was obtained by the following method.
[0116]
[0117] Take a 25mL dry reaction bottle and protect it with argon, add the crude intermediate S-8a (1.2g, 2.5mmol) and anhydrous dichloromethane (10mL) to the reaction bottle respectively, cool to -70°C, and slowly add 3M iso Propylmagnesium bromide (3.3 mL, 10 mmol). After the reaction is completed, add saturated ammonium chloride solution to quench, add dichloromethane to extract and separate the liquids, dry the oil phase over magnesium sulfate, filter and spin dry. The catalyst 1c (550mg, 41%) was obtained by using ethyl acetate:petroleum ether=1:20 to pass through the column.
[0118] colorless liquid, 1 H NMR (400MHz, DMSO-d6) δ7.69(s, 1H), 7.40-7.11(m, 17H), 6.97-6.94(m, 1H), 5.15(t, J=9.6Hz, 1H), 4.74( s,1H),4.47(t,J=9.2Hz,1H),3.81(t,J=8.5Hz,1H),2.69(d,J=4.8Hz,1H),1.82-1.73(m,2H), 0.91(d, J=6.4Hz, 3H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com