2-bromine-5-nitro-1,2,3,4-tetrahydro-1,4-methano-naphthalene-9-phenol and preparation method thereof
A technology of endomethylene and nitro, applied in 2-bromo-5-nitro-1,2,3,4-tetrahydro-1,4-endomethylene-naphthalene-9-phenol and its In the field of preparation, it can solve problems such as complex operation, poor stability of intermediates, and unsuitability for large-scale industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1 Synthesis of 2-bromo-5-nitro-1,2,3,4-tetrahydro-1,4-endomethylene-naphthalene-9-ol (IV)
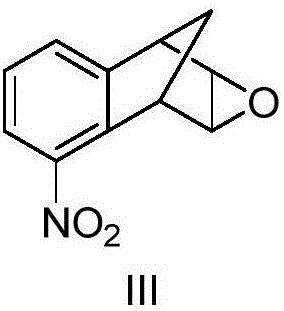
[0068] The molar ratio of the feed material is [2,3]-epoxy-5-nitro-1,2,3,4-tetrahydro-1,4-methano-naphthalene (III):48% hydrobromic acid Aqueous solution = 1:10
[0069] In a 500ml three-necked flask equipped with a mechanical stirrer and a thermometer, add 48% hydrobromic acid aqueous solution (177.2g, 1.05mol), and at 20°C, add dropwise [2,3]-epoxy-5-nitro-1 , 130ml of 2,3,4-tetrahydro-1,4-methano-naphthalene (III) (14.3g, 0.07mol) in dichloromethane solution, after 1.5h, the addition was completed, kept stirring for 5min, and analyzed by TLC Track responses. After the reaction was over, let stand to separate the liquids, wash the organic layer twice with 25ml of saturated sodium bicarbonate solution, then wash once with 40ml of saturated brine, separate the layers, dry and concentrate the organic layer to obtain a khaki solid, and wash the crude product with n-hexane...
Embodiment 2
[0071] Example 2 Synthesis of 2-bromo-5-nitro-1,2,3,4-tetrahydro-1,4-endomethylene-naphthalene-9-ol (IV)
[0072] The molar ratio of the feed material is [2,3]-epoxy-5-nitro-1,2,3,4-tetrahydro-1,4-methano-naphthalene (III):48% hydrobromic acid Aqueous solution = 1:5
[0073] 48% hydrobromic acid aqueous solution charging amount is 59.1g, all the other charging amount and operating process are with embodiment 1. 9.7 g of 2-bromo-5-nitro-1,2,3,4-tetrahydro-1,4-methano-naphthalene-9-ol (IV) was obtained, the yield was 49.2%, and the melting point was 132.1°C- 133.3°C, the physical and chemical data are the same as in Example 1.
Embodiment 3
[0074] Example 3 Synthesis of 2-bromo-5-nitro-1,2,3,4-tetrahydro-1,4-endomethylene-naphthalene-9-ol (IV)
[0075] The molar ratio of the feed material is [2,3]-epoxy-5-nitro-1,2,3,4-tetrahydro-1,4-methano-naphthalene (III):48% hydrobromic acid Aqueous solution = 1:20
[0076] 48% hydrobromic acid aqueous solution charging amount is 236.2g, all the other charging amount and operating process are with embodiment 1. 11.8 g of 2-bromo-5-nitro-1,2,3,4-tetrahydro-1,4-methano-naphthalene-9-ol (IV) was obtained, the yield was 59.6%, and the melting point was 132.1°C- 133.4°C, the physical and chemical data are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com