Chemically modified electrode for quantitative determination of folic acid and preparation method of electrochemical sensor
A quantitative detection and chemical modification technology, applied in the field of electrochemical sensing, can solve the problems of being susceptible to interferences, high detection limits, and complicated pre-processing of samples, and achieve enhanced electrocatalytic activity, detection performance, and good stability. , the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 Preparation of a chemical electrode for quantitative detection of folic acid
[0038] S1. Take 40 mg of multi-walled carbon nanotubes, add 1 mL of concentrated nitric acid and 3 mL of concentrated sulfuric acid, and then sonicate for 4 hours to obtain a suspension;
[0039] S2. Add ethanol to the above-mentioned suspension to 10 mL, and centrifuge; then add water and centrifuge until the pH value of the filtrate is neutral, and then dry the precipitate to obtain carboxylated multi-walled carbon nanotubes (MWCNTs);
[0040] S3. Take 4.5 mg of carboxylated multi-walled carbon nanotubes, add 3 mL of water for ultrasonic dispersion, and then add 0.19 g of glucose to form solution A;
[0041] S4. Take 0.026g of silver nitrate in 3mL of water and stir to dissolve, then add 0.5mL of NH 3 ·H 2 O forms solution B;
[0042] S5. Add solution B to solution A and stir for 0.5h. After aging for 2h, after centrifugation and washing, dry in an oven at 60°C for 12h to ob...
Embodiment 2
[0045] Embodiment 2 A kind of electrochemical sensor for quantitative detection of folic acid
[0046] The Ag / MWCNTs composite film modified electrode prepared in Example 1 was used as a working electrode, a saturated calomel electrode was used as a reference electrode, and a platinum wire electrode was used as an auxiliary electrode to assemble a three-electrode test system, and connected to an electrochemical workstation for folic acid Electrochemical sensors for quantitative detection.
Embodiment 3
[0047] Example 3 The electrical performance test of the electrochemical sensor used for the quantitative detection of folic acid
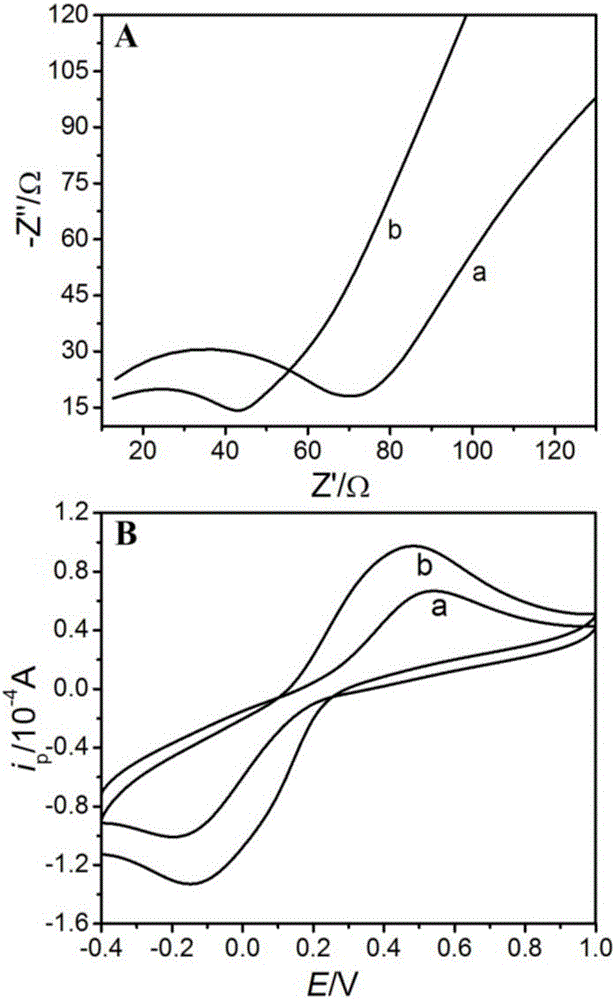
[0048] (1) Comparison of electron transfer performance of different electrodes
[0049] In the three-electrode test system prepared as in Example 2, the MWCNTs modified electrode (a) and the Ag / MWCNTs composite film modified electrode (b) prepared in Example 1 were used as working electrodes at 1.0mmol / L K 3 [Fe(CN) 6 ] The AC impedance and cyclic voltammetry tests were carried out in the bottom liquid mixed with 0.1mmol / L KCl, and the AC impedance test conditions were: frequency range 10 5 ~0.1HZ, amplitude 5mV, potential 0.18V; cyclic voltammetry test conditions are: potential range -0.4~1.0V, scanning speed 0.1V / s, the test results are as follows figure 2 . from figure 2 Visible electrochemical impedance is respectively 60 Ω and 30 Ω on two kinds of working electrodes of above-mentioned MWCNTs and Ag / MWCNTs composite film modification elec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| current | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com