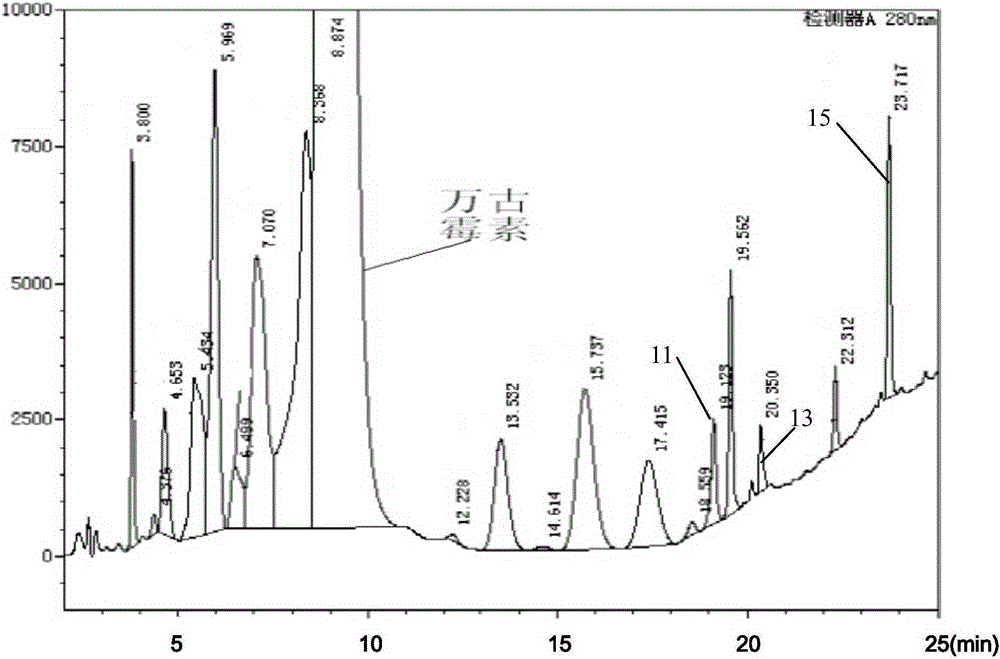
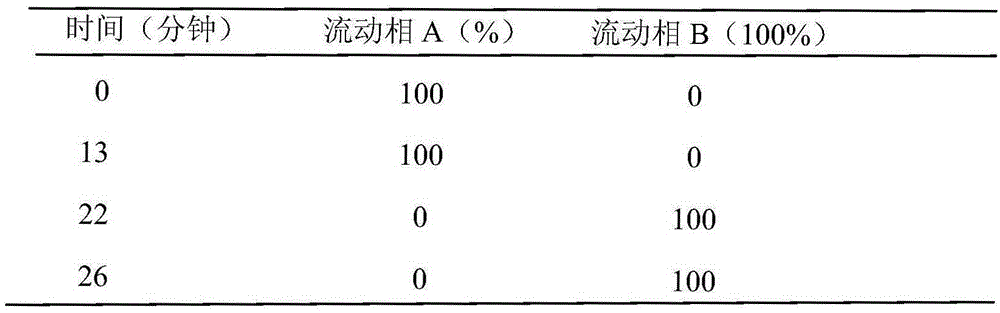
Preparation method of high purity samples of vancomycin hydrochloride impurities 11, 13, and 15
A vancomycin hydrochloride, high-purity technology is applied in the field of high-purity sample preparation, which can solve the problems of low content, high preparation cost, and consumption of a large amount of vancomycin hydrochloride, and achieve the effect of simple process and cost saving.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1. Take 10 g of vancomycin hydrochloride crystalline powder (purity detected by high pressure liquid chromatography is 95.1%), add 5% purified water to prepare a 52 g / L aqueous solution, and the solution volume is 192.4 mL;
[0048] 2. Adjust the pH of the solution to 4.15 with 2% oxalic acid solution, and keep it in a water bath at 40-42°C for 8 hours;
[0049] 3. Add 2% sodium bicarbonate solution to the solution after heat preservation to adjust the pH to 6.37;
[0050] 4. Perform FPDA13 resin chromatography on the solution after adjusting the pH. The column loading capacity of FPDA13 resin is 1500mL, and the flow rate is 2250mL / h.
[0051] 5. Use 4500mL of 0.03mol / L NH 4 Pre-wash the resin with Cl aqueous solution, the flow rate is 1200mL / h;
[0052] 6. Use 5500mL of 0.3mol / L NH 4 Cl aqueous solution is carried out eluting, and flow rate is 900mL / h, and 150mL collects a bottle, and sampling high-pressure liquid chromatography detects (detection method is the same...
Embodiment 2
[0058] 1. Take 10g of vancomycin hydrochloride crystalline powder (purity detected by high pressure liquid chromatography is 96.3%), add 5% purified water to prepare a 56g / L aqueous solution, and the solution volume is 178.6mL;
[0059] 2. Adjust the pH of the solution to 4.69 with 1% oxalic acid solution, and keep it in a water bath at 40-42°C for 9 hours;
[0060] 3. Add 1.5% sodium bicarbonate solution to the solution after heat preservation to adjust the pH to 6.93;
[0061] 4. Perform FPDA13 resin chromatography on the solution after adjusting the pH. The column loading capacity of FPDA13 resin is 1500mL, and the flow rate is 2250mL / h.
[0062] 5. Use 4500mL of 0.03mol / L NH 4 Pre-wash the resin with Cl aqueous solution, the flow rate is 1100mL / h
[0063] 6. Use 5500mL of 0.3mol / L NH 4 Cl aqueous solution was used for elution, the flow rate was 1000mL / h, 150mL was collected in one bottle, and samples were detected by high-pressure liquid chromatography, and the analysis s...
Embodiment 3
[0069] 1. Take 10g of vancomycin hydrochloride crystalline powder (purity detected by high pressure liquid chromatography is 95.3%), add 5% purified water to prepare a 60g / L aqueous solution, and the solution volume is 166.7mL;
[0070] 2. Adjust the pH of the solution to 5.86 with 1.5% oxalic acid solution, and keep it in a water bath at 40-42°C for 8.5 hours;
[0071] 3. Add 1.5% sodium bicarbonate solution to the solution after heat preservation to adjust the pH to 6.21;
[0072] 4. Perform FPDA13 resin chromatography on the solution after adjusting the pH. The column loading capacity of FPDA13 resin is 1500mL, and the flow rate is 1500mL / h.
[0073] 5. Use 4500mL of 0.03mol / L NH 4 Pre-wash the resin with Cl aqueous solution, the flow rate is 1100mL / h
[0074] 6. Use 5500mL of 0.3mol / L NH 4 Cl aqueous solution was used for elution, the flow rate was 900mL / h, 150mL was collected in one bottle, and samples were detected by high-pressure liquid chromatography, and the analy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com