Synthetic method of polyhydroxy substituted coumestrol natural product
A technology of coumestrol and natural products, which is applied in the field of synthesis of polyhydroxyl substituted coumestrol natural products, can solve the problems of low yield and long reaction steps, and achieve simple route, simple operation and atom economy high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
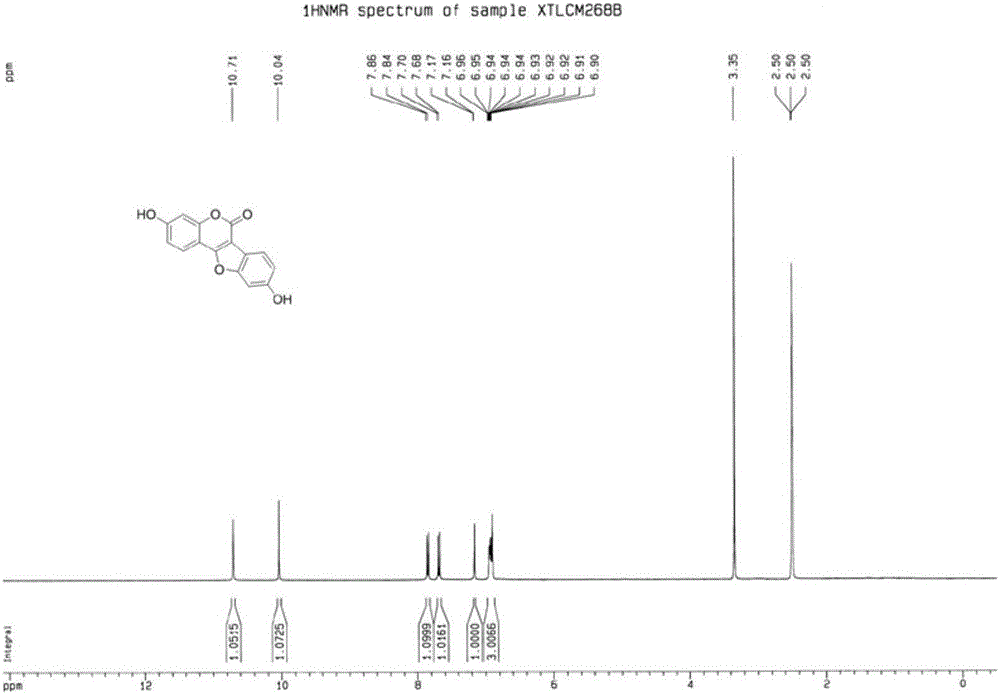
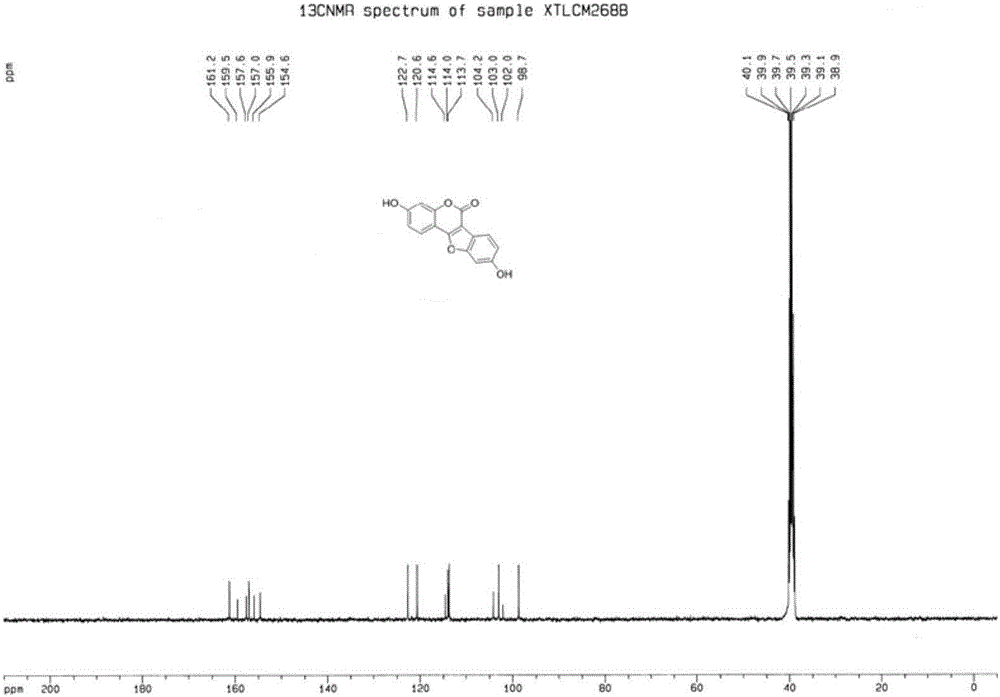
[0032] (1) Preparation of 3-(2-bromo-4-hydroxyphenyl)-7-hydroxycoumarin (structural formula 3a)
[0033] In a 50mL round bottom flask, add 2-bromo-4-hydroxyphenylacetic acid (25mmol, 5.78g), 2,4-dihydroxybenzaldehyde (25mmol, 3.45g), triethylamine (50mmol, 5.06g), ethyl Anhydride (10 mL), stirred, heated to 110° C. in an oil bath, and reacted for 7 hours. After the reaction was completed, the reaction solution was poured into 50 mL of ice water while it was hot, stirred, and solids precipitated out, filtered with suction, washed the filter cake with water, collected the filter cake, and obtained a light brown solid after drying, which was 3-(2-bromo-4-hydroxyl Phenyl)-7-hydroxycoumarin, weight 7.83g, yield 94%. 1 H NMR (DMSO-d 6,400MHz,ppm)δ10.38(s,OH),9.94(s,OH),7.89(s,1H),7.57(d,J=8.8Hz,1H),7.26(d,J=8.4Hz,1H ),7.11(d,J=2.0Hz,1H),6.86(dd,J=2.0,8.8Hz,1H),6.83(dd,J=2.0,8.4Hz,1H),6.78(d,J=2.0Hz ,1H); 13 C NMR (DMSO-d 6 ,100MHz,ppm)δ161.3,159.6,158.2,155.2,143.0,132.5,129.9...
Embodiment 2
[0037] (1) Preparation of 3-(2-bromo-4-hydroxyphenyl)-7-hydroxycoumarin (structural formula 3a)
[0038] In a 50mL round bottom flask, add 2-bromo-4-hydroxyphenylacetic acid (25mmol, 5.78g), 2,4-dihydroxybenzaldehyde (25mmol, 3.45g), triethylamine (25mmol, 2.53g), ethyl Acid anhydride (10 mL), stirred, heated to 110° C. in an oil bath, and reacted for 7 hours. After the reaction is completed, the reaction solution is poured into 50 mL of ice water while it is hot, stirred, and solids are precipitated, filtered with suction, washed with water, collected, and dried to obtain a light brown solid, which is 3-(2-bromo-4-hydroxyl Phenyl)-7-hydroxycoumarin, weight 6.91g, yield 83%.
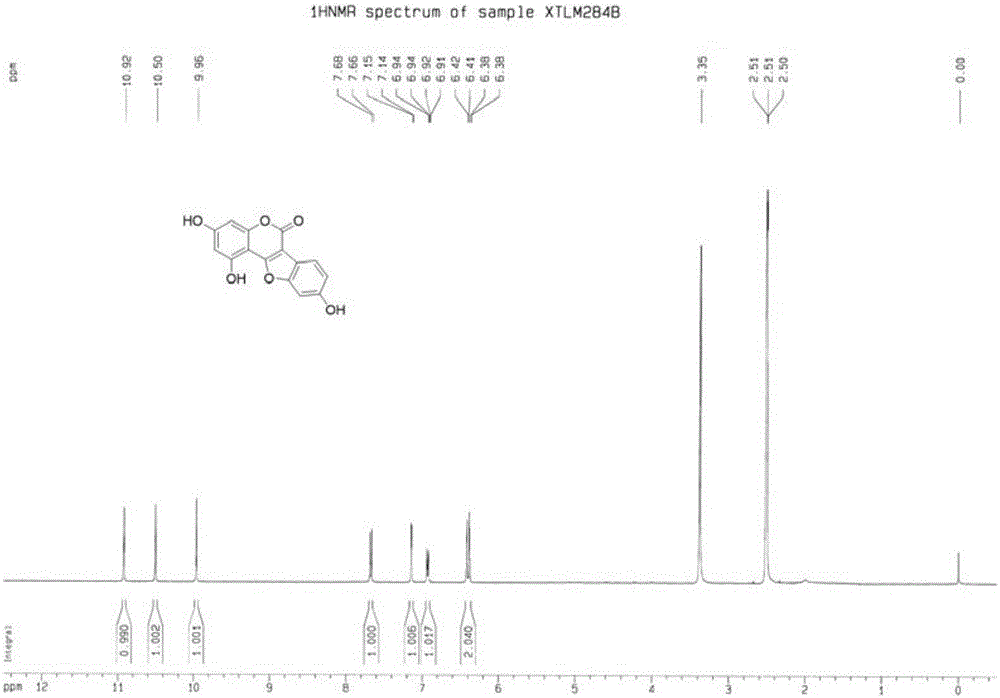
[0039] (2) Preparation of 3,9-dihydroxy-6H-benzofuro[3,2-c]chroman-6-one (structural formula 4a)
[0040] In a 50mL microwave reaction flask, add 3-(2-bromo-4-hydroxyphenyl)-7-hydroxycoumarin (5mmol, 1.67g), basic copper carbonate (1mmol, 0.24g), 1,10 -O-phenanthroline (1mmol, 0.19g), potassium hydrox...
Embodiment 3
[0042] (1) Preparation of 3-(2-bromo-4-hydroxyphenyl)-7-hydroxycoumarin (structural formula 3a)
[0043] In a 50mL round bottom flask, add 2-bromo-4-hydroxyphenylacetic acid (25mmol, 5.78g), 2,4-dihydroxybenzaldehyde (25mmol, 3.45g), triethylamine (75mmol, 7.59g), ethyl Acid anhydride (10 mL), stirred, heated to 110° C. in an oil bath, and reacted for 7 hours. After the reaction is completed, the reaction solution is poured into 80 mL of ice water while it is hot, stirred, and solids are precipitated, filtered with suction, washed with water, collected, and dried to obtain a light brown solid, which is 3-(2-bromo-4-hydroxyl Phenyl)-7-hydroxycoumarin, weight 7.16g, yield 86%.
[0044] (2) Preparation of 3,9-dihydroxy-6H-benzofuro[3,2-c]chroman-6-one (structural formula 4a)
[0045] In a 50mL microwave reaction flask, add 3-(2-bromo-4-hydroxyphenyl)-7-hydroxycoumarin (5mmol, 1.67g), copper oxide (1mmol, 0.08g), 1,10-o- Phenanthroline (1mmol, 0.19g), potassium hydroxide (50mmo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com