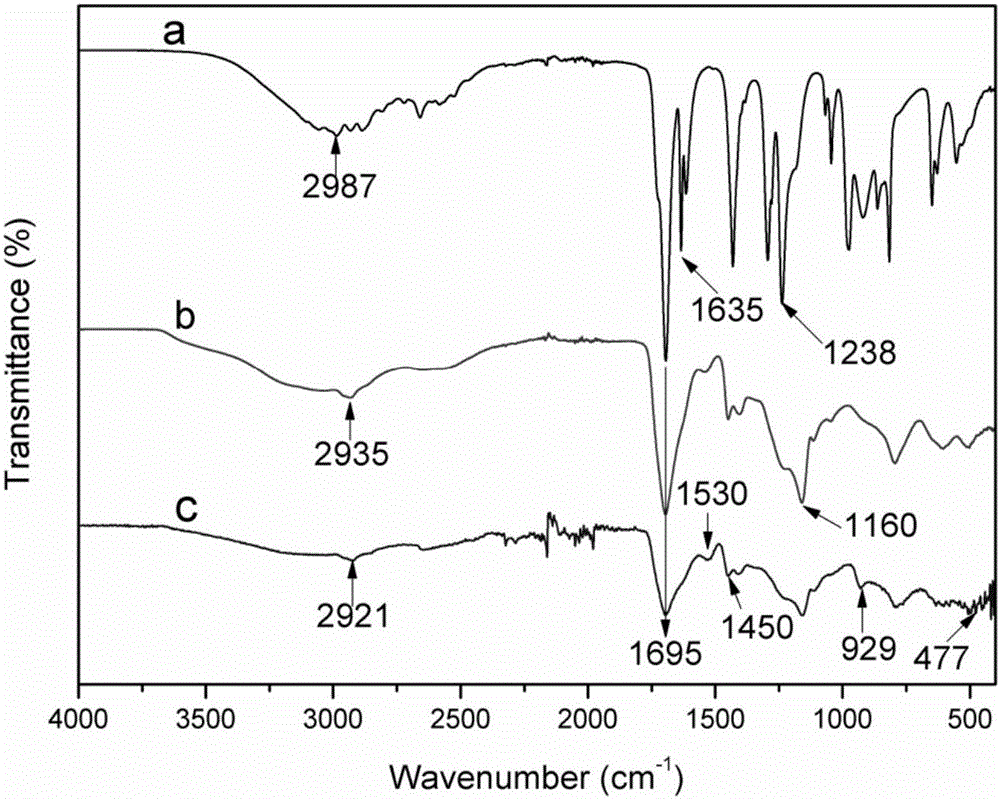
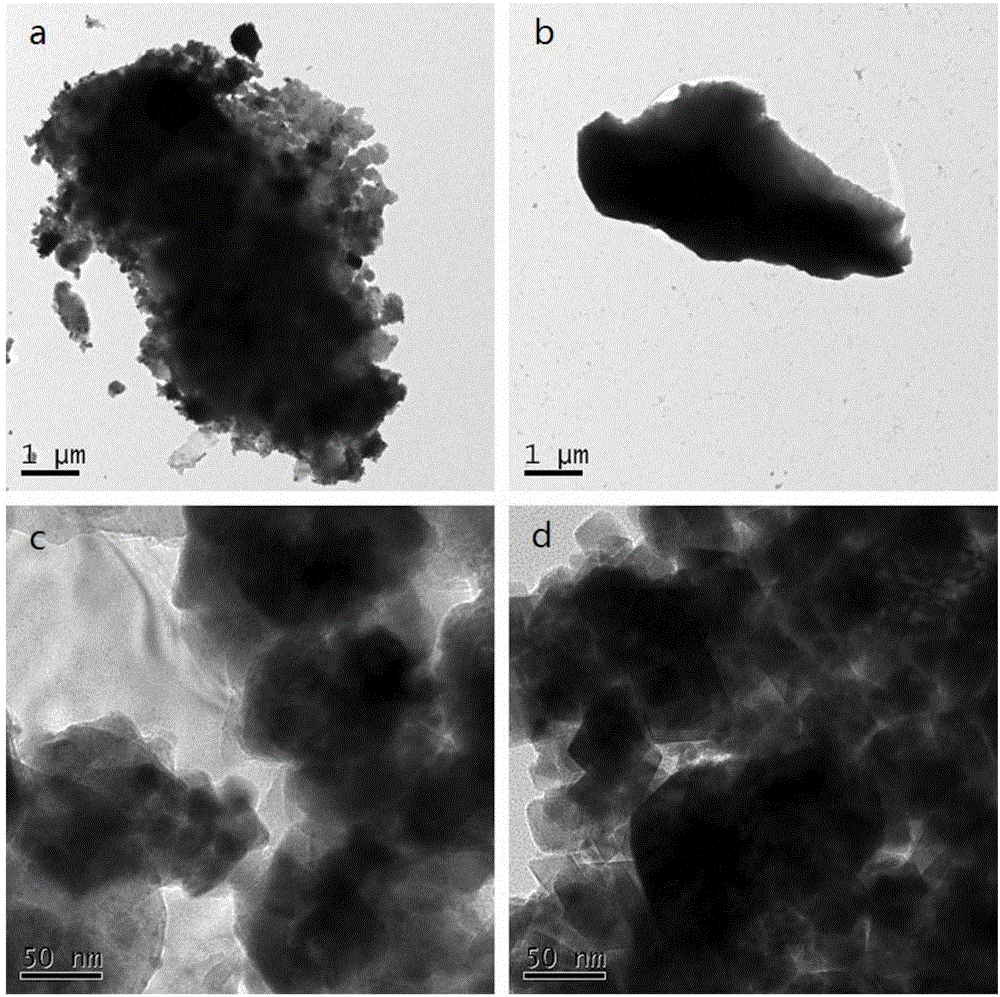
Preparation method and application of polyacrylic acid hydrogel adsorbing material
An adsorption material, polyacrylic acid technology, applied in chemical instruments and methods, adsorption water/sewage treatment, water pollutants, etc., can solve the problems of poor mechanical strength, low adsorption performance, long polymerization time, etc., and achieve the conditions required for the reaction Less, effective and fast removal, simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A preparation method of a polyacrylic acid hydrogel adsorption material is as follows: respectively preparing an acrylic acid solution, an ammonium persulfate solution and a sodium hydrogen sulfite solution, and dissolving N,N'-methylenebisacrylamide in the acrylic acid solution, Then add ammonium persulfate solution and sodium bisulfite solution, add water to dilute to obtain a mixed solution, in the mixed solution, the mass fraction of acrylic acid is 30%, and the mass fraction of N,N'-methylenebisacrylamide is 1% , the mass fraction of ammonium persulfate is 0.42%, and the mass fraction of sodium bisulfite is 0.42%;
[0026] Then, the above mixed solution is stirred evenly, and the hydrogel is formed by standing for a polymerization reaction. The polymerization temperature of the polymerization reaction is 25° C. and the polymerization time is 2 hours. The obtained hydrogel is chopped and freeze-dried to obtain polyacrylic acid water. The gel adsorption material was ...
Embodiment 2
[0029] A preparation method of a polyacrylic acid hydrogel adsorption material is as follows: respectively preparing an acrylic acid solution, an ammonium persulfate solution and a sodium hydrogen sulfite solution, and dissolving N,N'-methylenebisacrylamide in the acrylic acid solution, Then add ammonium persulfate solution and sodium bisulfite solution, add water to dilute to obtain a mixed solution, in the mixed solution, the mass fraction of acrylic acid is 28%, and the mass fraction of N,N'-methylenebisacrylamide is 1.2% , the mass fraction of ammonium persulfate is 0.35%, and the mass fraction of sodium bisulfite is 0.35%;
[0030] Then, the above mixed solution was stirred evenly, and the hydrogel was formed by standing for polymerization reaction. The polymerization temperature of the polymerization reaction was 23° C., and the polymerization time was 2.5 h. The obtained hydrogel was chopped and freeze-dried to obtain polyacrylic acid. The hydrogel adsorption material w...
Embodiment 3
[0033] A method for preparing a polyacrylic acid adsorption material is as follows: respectively preparing an acrylic acid solution, an ammonium persulfate solution and a sodium hydrogen sulfite solution, dissolving N,N'-methylenebisacrylamide in the acrylic acid solution, and then adding a Ammonium sulfate solution and sodium bisulfite solution are diluted with water to obtain a mixed solution. In the mixed solution, the mass fraction of acrylic acid is 32%, the mass fraction of N,N'-methylenebisacrylamide is 0.8%, and the mass fraction of persulfuric acid is 0.8%. The mass fraction of ammonium is 0.45%, and the mass fraction of sodium bisulfite is 0.45%;
[0034] Then, the above mixed solution is stirred evenly, and a hydrogel is formed by standing for a polymerization reaction. The polymerization temperature of the polymerization reaction is 27° C., and the polymerization time is 2-2.5 h. The obtained hydrogel is chopped and freeze-dried to obtain Polyacrylic acid hydrogel ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com