Nonaqueous electrolytic solution including ester having 3,3,3-trifluoropropionate group and nonaqueous electrolyte battery using same
A non-aqueous electrolyte, trifluoropropionate technology, applied in non-aqueous electrolyte batteries, non-aqueous electrolytes, organic electrolytes and other directions, can solve the problems of reduced battery characteristics, low stability of polymer coating, and deterioration of battery capacity, etc. Achieve the effect of suppressing gas generation, excellent oxidation resistance, and suppressing capacity deterioration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[0062]
[0063] In a 50 mL reaction apparatus equipped with a fractionator, 6.10 g (98.3 mmol) of ethylene glycol and 28.2 g (220 mmol) of 3,3,3-trifluoropropionic acid were mixed. 0.21 g (2.1 mmol) of 95% sulfuric acid was added to the mixture, and the reaction was carried out while heating the temperature in the reaction apparatus to 100° C. under reduced pressure (30 kPa) and distilling off water. After reacting for 7 hours, it was cooled to room temperature, and 50 mL of water was added to the reaction product, followed by stirring. The organic phase was separated and washed with 100 mL of 5% sodium bicarbonate water to obtain a crude product of ethylene bis(3,3,3-trifluoropropionate) as a colorless clear liquid. Yield 24.3 g, 85% yield. As a result of measurement by gas chromatography, the gas chromatogram area of ethylene bis(3,3,3-trifluoropropionate) was 97.2%. The crude product was purified by precision distillation under reduced pressure (Snyder 5 sphere, 0.25 ...
manufacture example 2
[0069]
[0070] In a 50 mL reaction apparatus equipped with a fractionator, 8.11 g (90.0 mmol) of 1,4-butanediol and 23.9 g (187 mmol) of 3,3,3-trifluoropropionic acid were mixed. 0.16 g (1.7 mmol) of 95% sulfuric acid was added to the mixture, and the temperature in the reaction apparatus was heated to 120° C. under reduced pressure (40 kPa), and the reaction was carried out while distilling off water. After reacting for 8 hours, it was cooled to room temperature, and 50 mL of water was added to the reaction product, followed by stirring. The organic phase was separated and washed with 100 mL of 5% sodium bicarbonate water to obtain a crude product of tetramethylenebis(3,3,3-trifluoropropionate) as a light brown liquid. Yield 23.4 g, 84% yield. As a result of measurement by gas chromatography, the gas chromatogram area of tetramethylenebis(3,3,3-trifluoropropionate) was 97.3%. The crude product was purified by precision distillation under reduced pressure (Snyder 5 sphe...
Embodiment 1
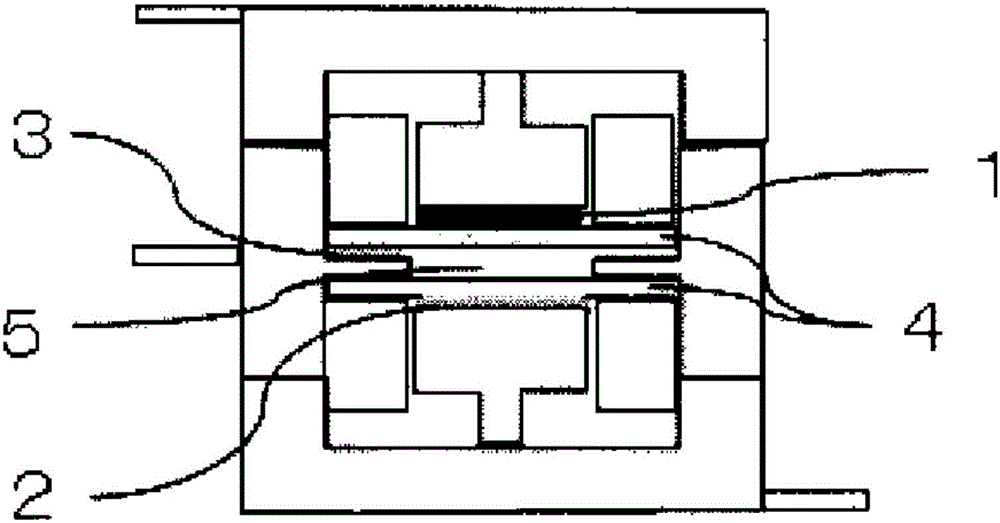
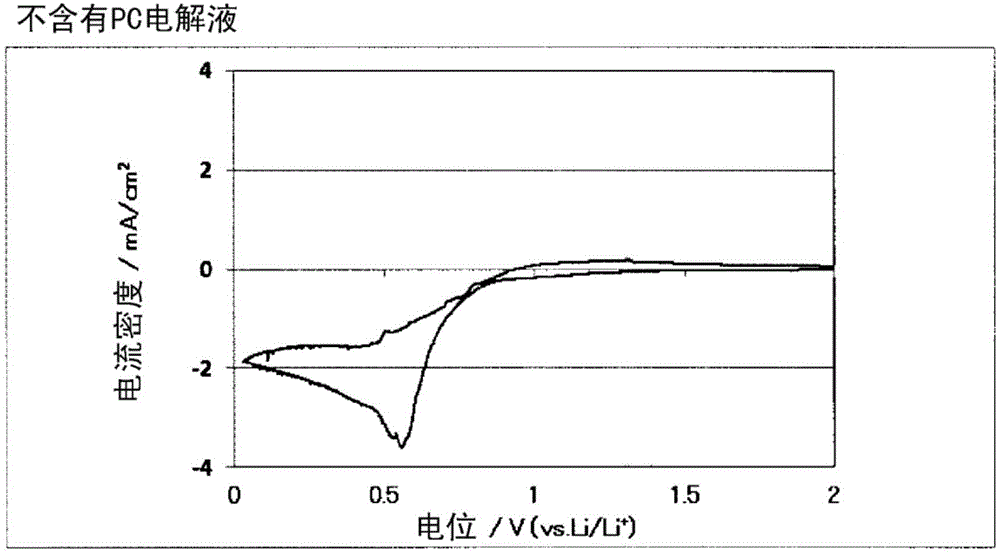
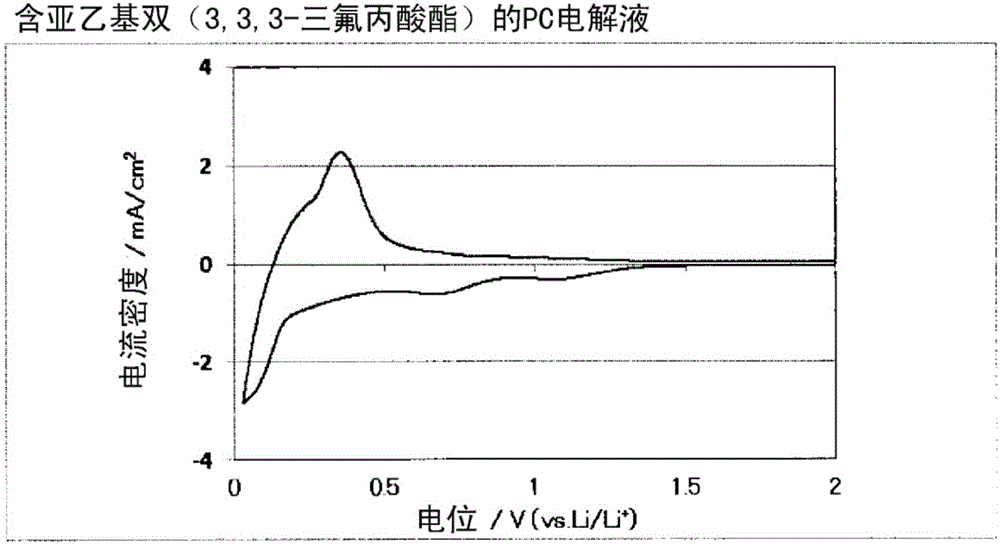
[0076] Ethylene bis(3,3,3-trifluoropropionate) represented by formula 2 produced in Production Example 1 was mixed with 97 wt% of propylene carbonate (PC) so as to be 3 wt%, to prepare a non-aqueous system solvent. Lithium hexafluorophosphate LiPF as electrolyte 6 It was dissolved in this non-aqueous solvent at a ratio of 1 mol / L to prepare a non-aqueous electrolytic solution. Using this non-aqueous electrolyte, making figure 1 The three-electrode test cell shown.
[0077] In the above-mentioned three-electrode battery cell, a sealed three-electrode battery cell manufactured by Keihin Rika Kogyo Co., Ltd. was used, and a natural graphite-coated electrode sheet (negative electrode single layer) manufactured by Piotrek Co., Ltd. was used as the working electrode 1 and cut to a specified size. For the obtained material, lithium metal was used for the counter electrode 2 and the reference electrode 3 , and the separator 4 was interposed between these electrodes, and immersed i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com