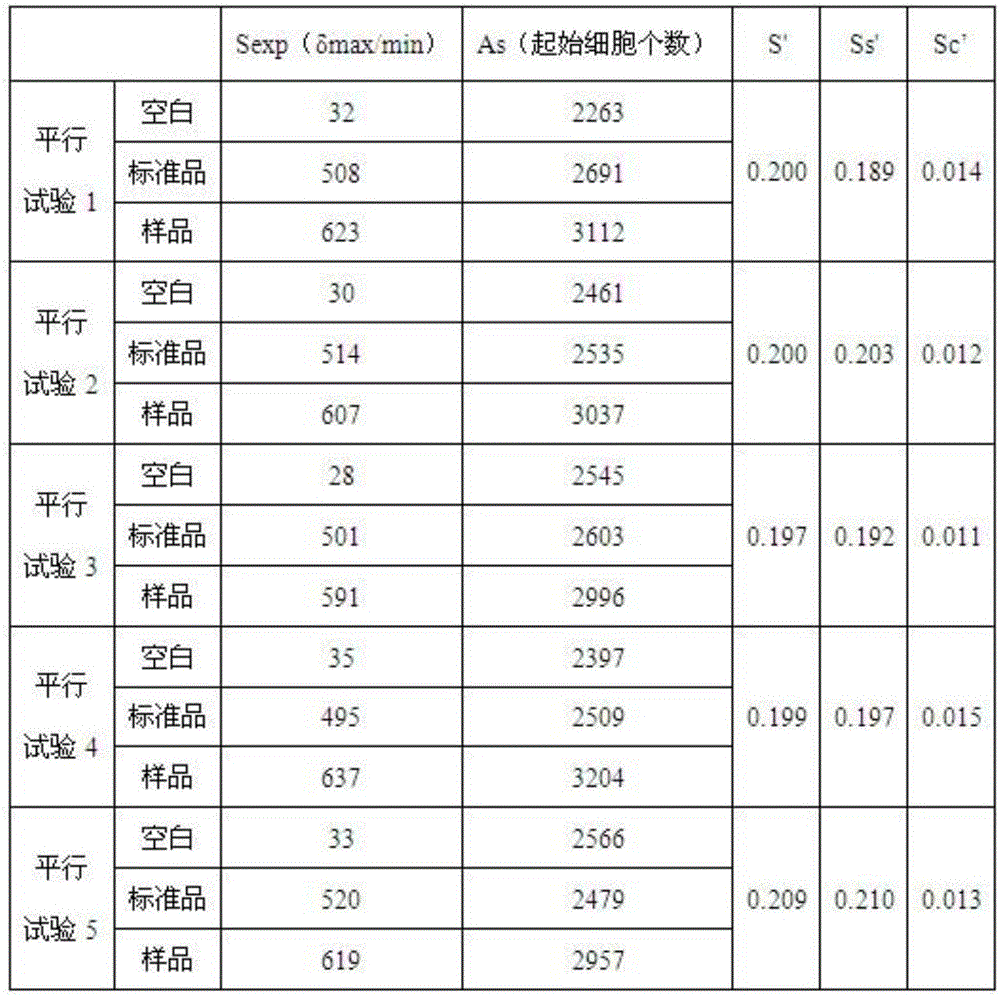
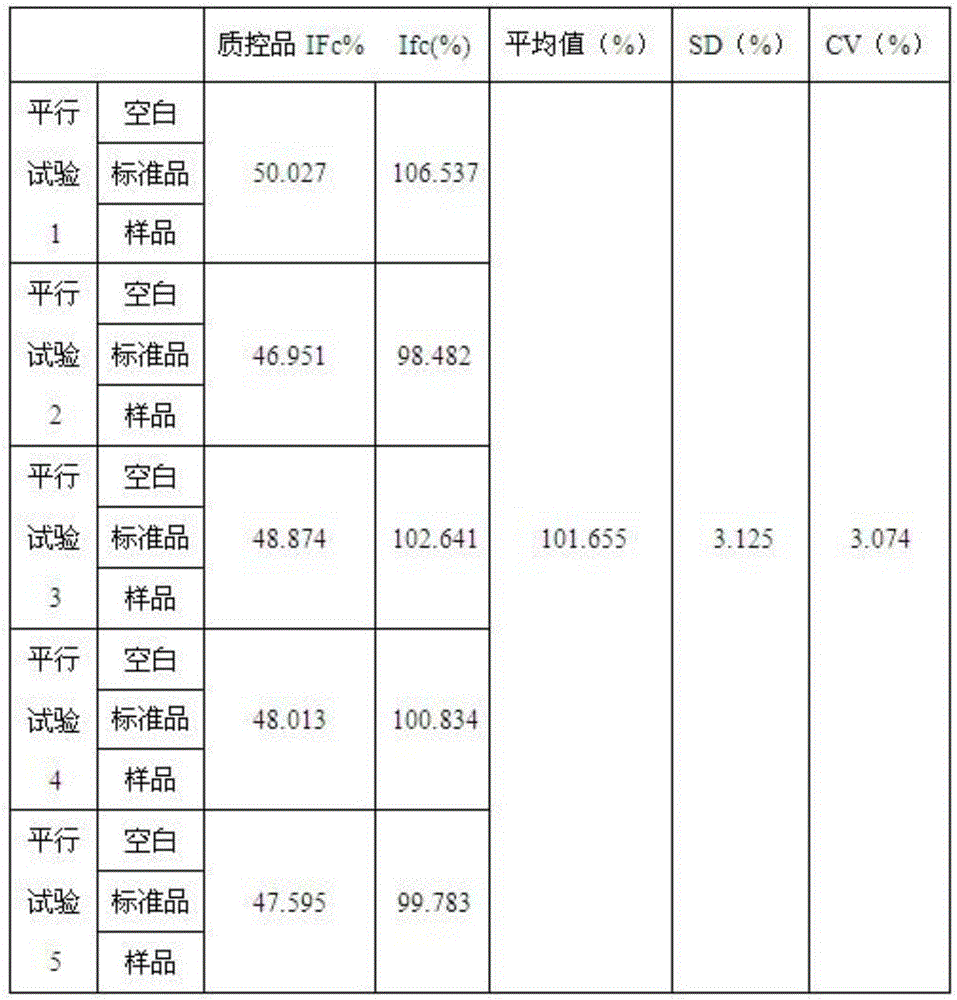
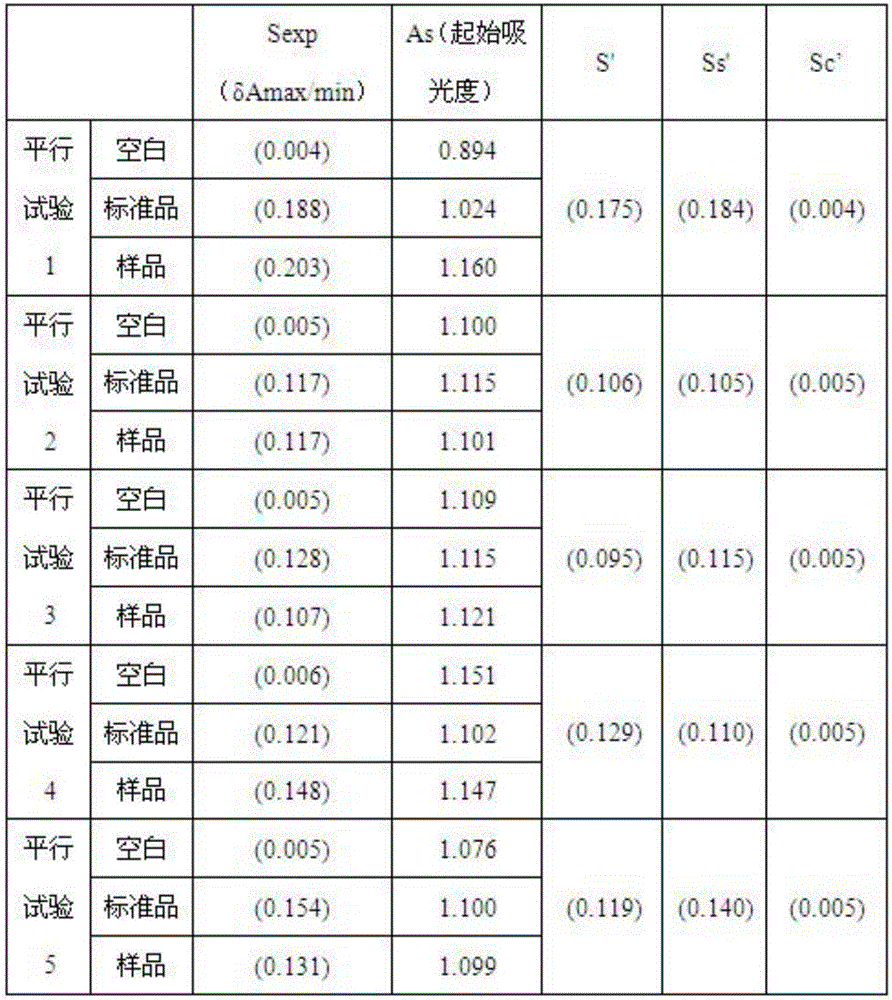
Human intravenous immunoglobulin Fc fragment activity detection method
A technology for human immunoglobulin and activity detection, which is applied in measurement devices, biological tests, material inspection products, etc., can solve the problems of high reagent cost, fluctuation of experimental data, low throughput, etc. The effect of good blank stability and high resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] 1. Material preparation:
[0079] 1.1 Reagents:
[0080] NaH 2 PO 4 2H 2 O; Na 2 HPO 4 12H 2 O; NaCl; CaCl 2 ; MgCl 2 ·6H 2 O; NaOH; concentrated hydrochloric acid; barbital sodium; bovine albumin (Sigma); tannic acid; Fc standard.
[0081] 1.2 Consumables:
[0082] 15ml pyrogen-free tube; 5ml pyrogen-free tube; 2ml pyrogen-free tube (sterile cryopreservation tube); 1.5ml EP tube; 50ml syringe; 5ml syringe; cell counting plate; disposable vacuum blood collection tube.
[0083] 1.3 Equipment:
[0084] Desktop refrigerated centrifuge; constant temperature water bath; desktop centrifuge; cell counter.
[0085] 2. Preparation before experiment:
[0086] 2.1 Collection of fresh blood:
[0087] Collect 3-5ml of fresh type O blood in a disposable vacuum blood collection tube.
[0088] 2.2 Reagent preparation:
[0089] 1) 0.5M NaOH: Weigh 2g of NaOH, add 99.5ml of purified water to dissolve.
[0090] 2) PBS buffer (pH7.2): Na 2 HPO 4 12H 2 O 1.285g; NaH 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com