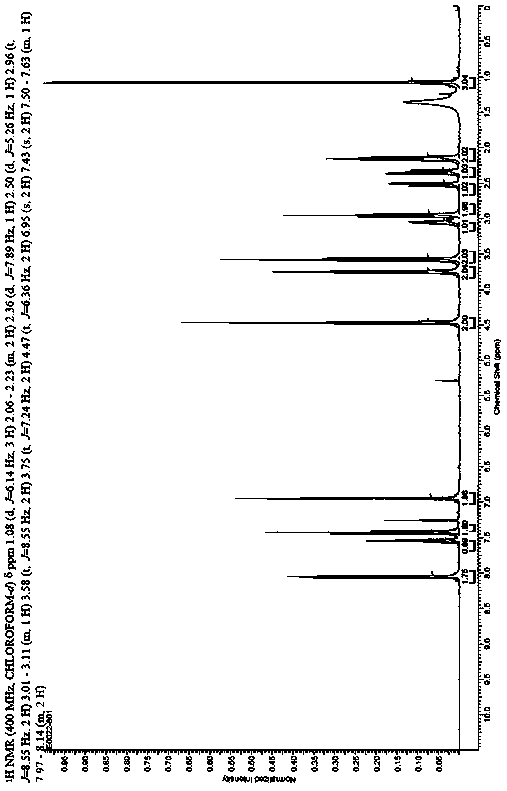
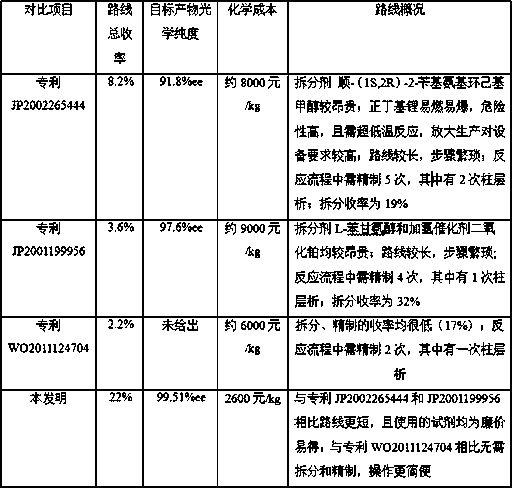
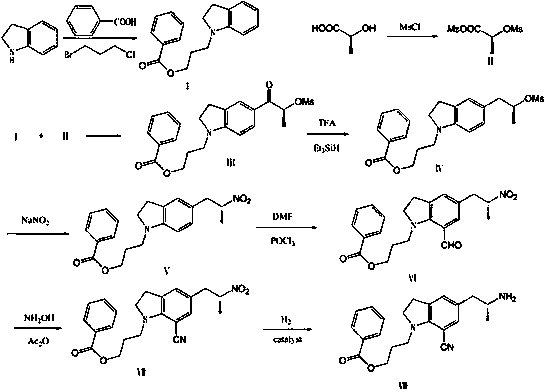
A kind of preparation method for the intermediate of synthetic silodosin
A technology for silodosin and intermediates, which is applied in the synthesis field of pharmaceutical intermediates, can solve the problems of poor reductive amination selectivity, unfavorable enlarged production and high production costs, and achieves the effects of simple operation, loss avoidance and cost reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of compound I, 1-(3-benzoyloxypropyl)indoline.
[0039] Add 40.2g of benzoic acid, 135ml of DMF, 45.9ml of triethylamine and 32.5ml of 1-chloro-3-bromopropane to the reaction flask, stir at 25°C for 12 hours, heat up to 50°C and stir for 3 hours, add 34.9ml of indoline, 45.9 ml of triethylamine, react at 100°C for 6 hours. Cool to room temperature, add 270ml of water, extract twice with ethyl acetate, combine the ethyl acetate layers, wash with saturated sodium bicarbonate and saturated brine in turn, add 3mol / L dilute hydrochloric acid to extract to the water layer, add saturated sodium carbonate solution Adjust the pH=8-9, add dichloromethane to extract twice, wash with saturated brine, dry over anhydrous sodium sulfate, and recover the dry solvent under reduced pressure to obtain 69.7g of 1-(3-phenoxypropyl)indoline; Yield: 78%, purity: 98.66%.
Embodiment 2
[0040] Example 2: Preparation of Compound II.
[0041] Add 600ml of anhydrous dichloromethane, 19.6g of L-lactic acid and 50.8g of triethylamine to the reaction bottle, cool down to -20°C, add 55.8g of methanesulfonyl chloride dropwise, control the temperature not to exceed -10°C, and -10 ℃ for 1 hour. The next reaction can be carried out directly without treatment; the intermediates are not easy to separate, and the yield is inconvenient to calculate.
Embodiment 3
[0042] Example 3: Preparation of compound III, namely (S)-[1-(3-benzoyloxypropyl)indolin-5-yl]-2-methanesulfonyloxypropyl-1-one.
[0043]Add 55.6 g of 1-(3-benzoyloxypropyl)indoline prepared in Example 1 in batches to the reaction solution of Compound II prepared in Example 2, and control the temperature not to exceed 0°C. After the addition, 0 Stir at 0°C for 15 minutes, cool down to -10°C, add 30.2g of anhydrous aluminum trichloride in batches, control the temperature not to exceed 0°C, stir at 0°C for 30 minutes after the addition, raise the temperature to 25°C for reaction, and the raw materials are completely reacted Finally, add the reaction mixture to 2000ml of ice-water mixture, add potassium carbonate solution to adjust ph=7-8, filter, separate the filtrate, extract the water layer with 400ml of dichloromethane, combine the dichloromethane layers, dry, and recover under reduced pressure. solvent, to obtain 64.8g of reddish-brown oily substance, namely (S)-[1-(3-phenox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com