O-phenylenediamine schiff base derivative as well as preparation method and application thereof
A technology of o-phenylenediamine and nitro-o-phenylenediamine, applied in the field of preparation of o-phenylenediamine Schiff base derivatives and o-phenylenediamine Schiff base derivatives, can solve difficult separation and purification, long reaction time , many side reactions and other problems, to achieve the effect of good chemical stability, short reaction time, easy operation and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a 25ml hydrothermal reaction kettle, add 10ml of water (that is, the filling degree is 40%), and 3mmol of 4-nitro-o-phenylenediamine and 3mmol of benzaldehyde, and react at 120° C. for 20 minutes. After the reaction is completed, the reaction kettle is cooled to room temperature, and the reaction product is filtered, dried, recrystallized and dried to obtain the target product.
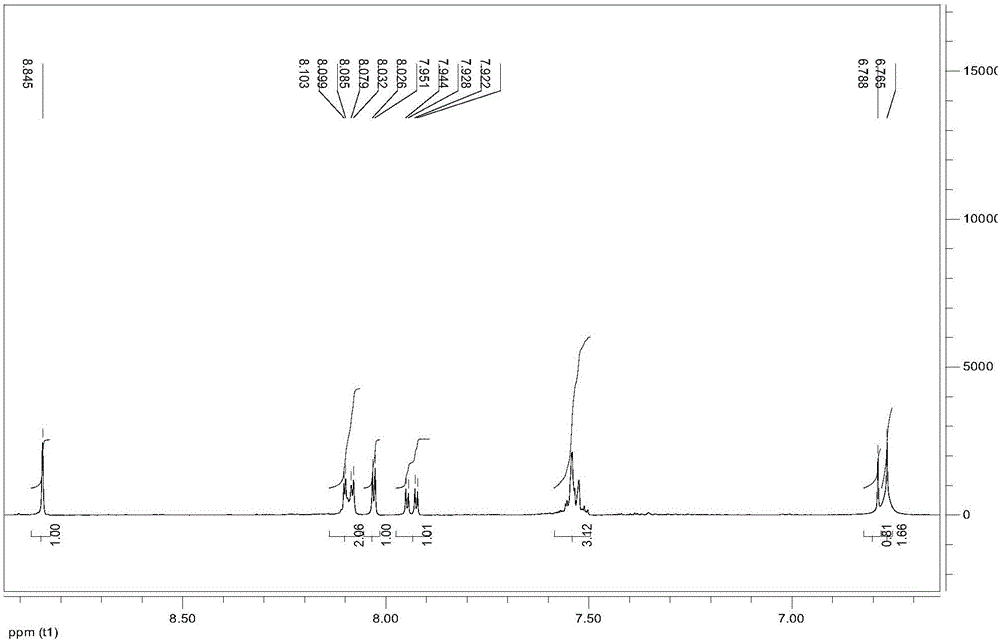
[0029] figure 1 is the o-phenylenediamine Schiff base derivative obtained in Example 1 of the present invention 1 H-NMR spectrum.
[0030] 1 H-NMR (400MHz, DMSO-d 6 )δ: 8.85(s,1H), indicating that the Schiff base has been successfully prepared, 8.03-8.10(m,2H), 8.02(m,1H), 7.89(m,1H), 7.49-7.58(m,3H), 6.79 (m, 1H), 6.76 (m, 2H).
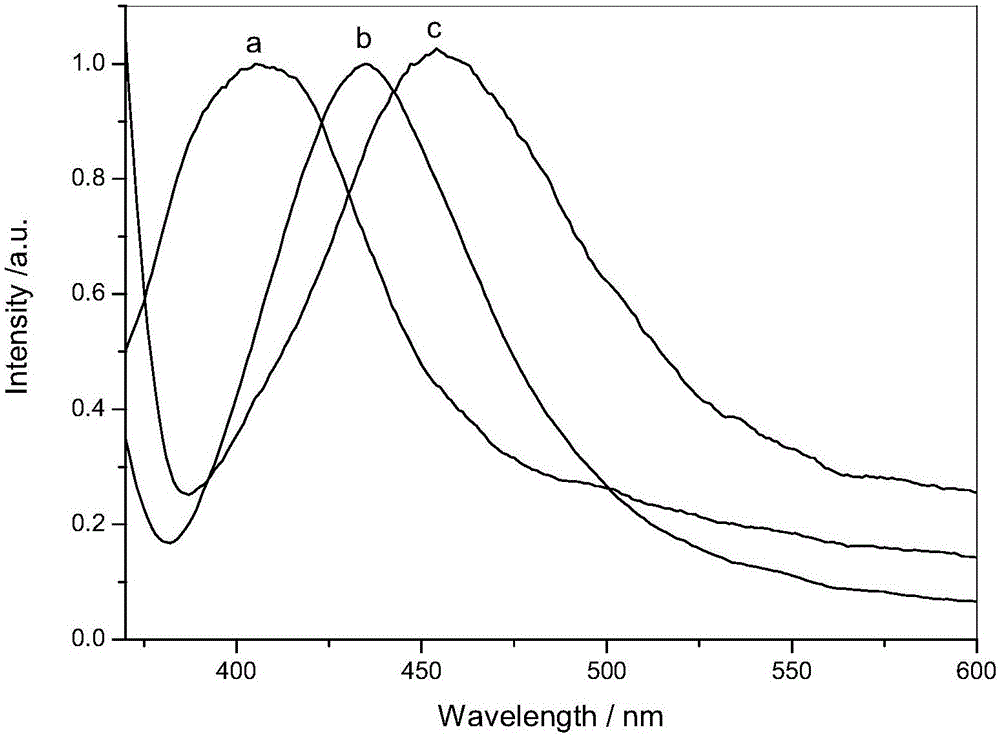
[0031] figure 2 It is the o-phenylenediamine Schiff base derivative obtained in Example 1 of the present invention (concentration is 1×10 -5 mol / L) in cyclohexane, dichloromethane, normalized fluorescence emission spectrum in absolute ethanol (excitation wa...
Embodiment 2
[0033] In a 25ml hydrothermal reaction kettle, add 10ml of water (that is, the filling degree is 40%), and 2mmol of 4-nitro-o-phenylenediamine and 2mmol of benzaldehyde, and react at 120° C. for 40 minutes. After the reaction is finished, the reactor is cooled to room temperature, and the reaction product is filtered, dried, recrystallized and dried to obtain the target product.
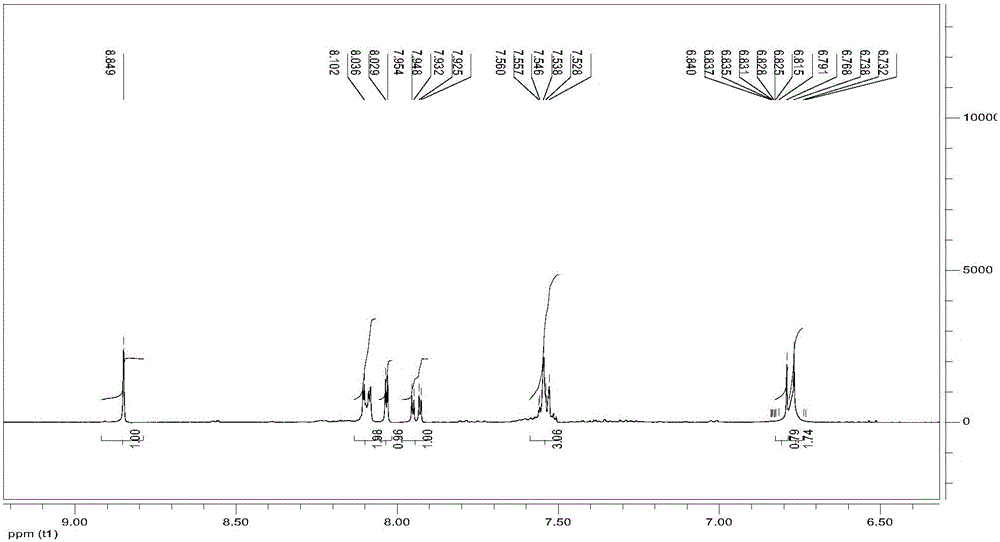
[0034] image 3 is the o-phenylenediamine Schiff base derivative obtained in Example 2 of the present invention 1 H-NMR spectrum.
[0035] 1 H-NMR (400MHz, DMSO-d 6 )δ: 8.82(s,1H), indicating that the Schiff base has been successfully prepared, 8.10(m,2H), 8.02(m,1H), 7.92(m,1H), 7.52(m,3H), 6.81(m, 1H), 6.73-6.79 (m, 2H).
Embodiment 3
[0037] In a 50ml hydrothermal reaction kettle, add 10ml of water (ie, the filling degree is 20%), and 3mmol of 4-nitro-o-phenylenediamine and 3mmol of benzaldehyde, and react at 100°C for 90 minutes. After the reaction is finished, the reactor is cooled to room temperature, and the reaction product is filtered, dried, recrystallized and dried to obtain the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com