Desonide film coating agent and preparation method thereof
A coating agent, desonide technology, applied in anti-inflammatory agents, pharmaceutical formulations, non-central analgesics, etc., can solve adverse effects on product quality and stability, easy hydrolysis or degradation by other means, desonide To avoid problems such as unstable chemical properties, to prevent short drug adhesion time and loss, easy to control, and ensure drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
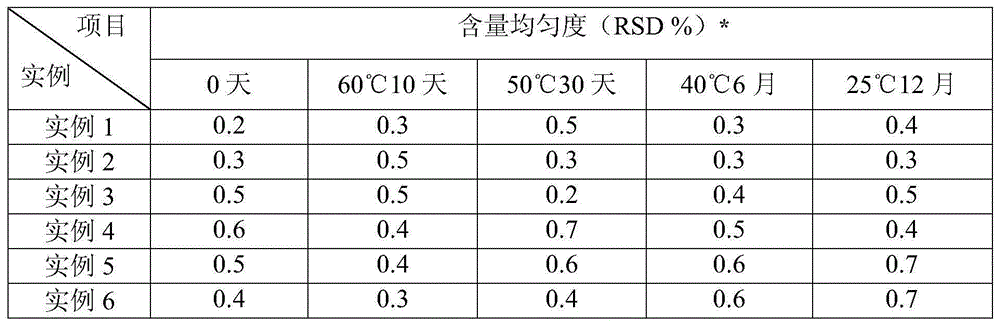
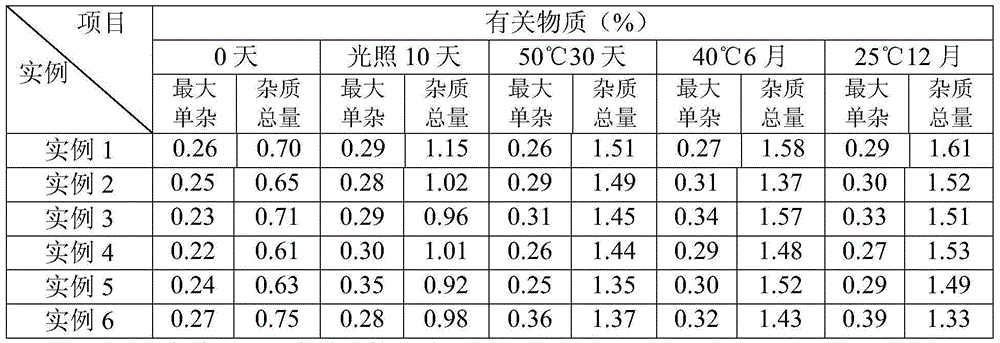
Embodiment 1
[0030] The prescription of desonide coating agent of the present invention consists of:
[0031] Desonide 0.5g Polyvinyl alcohol 05-88 100.0g Titanium dioxide 3.0g Carbomer 981 5.0g glycerin 50.0g Dimethyl sulfoxide 50.0g 2-Phenoxyethanol 5.0g
[0032] citric acid 0.4g sodium hydroxide 0.1g Water for Injection Fixed weight to 1000g
[0033] Preparation:
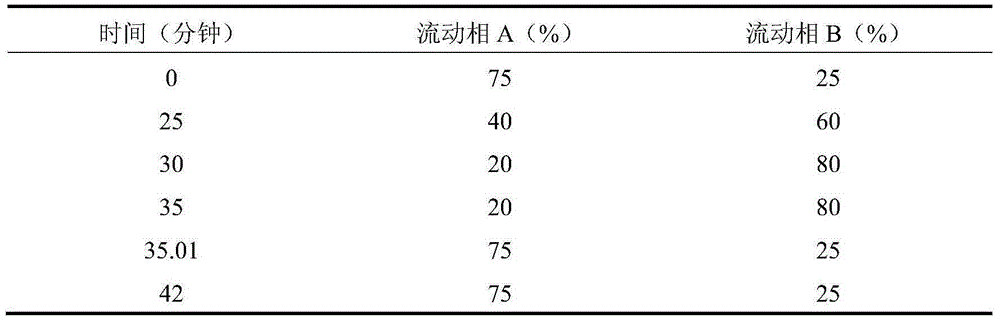
[0034] 1) Weigh the prescribed amount of polyvinyl alcohol 05-88 and add a certain amount of water for injection to swell for 24 hours to make a gel;
[0035] 2) Carbomer 981 was swollen with a certain amount of water for injection for 4 hours, and the active ingredient desonide was dissolved in dimethyl sulfoxide and 2-phenoxyethanol, then added to the carbomer solution and stirred to mix and disperse;
[0036] 3) Titanium dioxide (over 180 mesh) was added to 2) with glycerin to stir and moisten, fully stirred to mix and disperse, and added the p...
Embodiment 2
[0039] The prescription of desonide coating agent of the present invention consists of:
[0040] Desonide 0.5g Polyvinyl alcohol 17-88 90.0g Titanium dioxide 3.0g Sodium carboxymethyl cellulose 15.0g glycerin 50.0g dimethylformamide 20.0g Benzyl alcohol 5.0g ethanol 50.0g citric acid 0.35g sodium citrate 1.0g Water for Injection Fixed weight to 1000g
[0041] Preparation:
[0042] 1) Weigh the prescribed amount of polyvinyl alcohol 17-88, add a certain amount of water for injection to swell for 24 hours to make a gel;
[0043] 2) First dissolve the pH regulator with water for injection; then disperse carboxymethylcellulose sodium with a certain amount of water for injection above 80°C, add it to the pH regulator solution, stir and swell for 4 hours to form a gel;
[0044] 3) The active ingredient desonide is added to dimethylformamide and benzyl alcohol, stirred and dissolved, then added to 2), fully s...
Embodiment 3
[0048] The prescription of desonide coating agent of the present invention consists of:
[0049] Desonide 0.5g Polyvinyl alcohol 124 90.0g Zinc oxide 5.0g Hydroxypropylmethylcellulose 20.0g Propylene Glycol 50.0g Laurocaprazine 20.0g 2-Phenoxyethanol 7.0g ethanol 30.0g citric acid 0.35g sodium citrate 1.0g Water for Injection Fixed weight to 1000g
[0050] Preparation:
[0051] The preparation method refers to Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com