Use of set of biomarkers in preparation of colorectal cancer diagnostic reagents
A biomarker and colorectal technology, which is applied in the field of prediction or diagnosis of colorectal cancer, can solve the problem of undetermined biomarker colorectal cancer development stage information and guide treatment, etc., to achieve the effect of convenient individual examination and easy use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
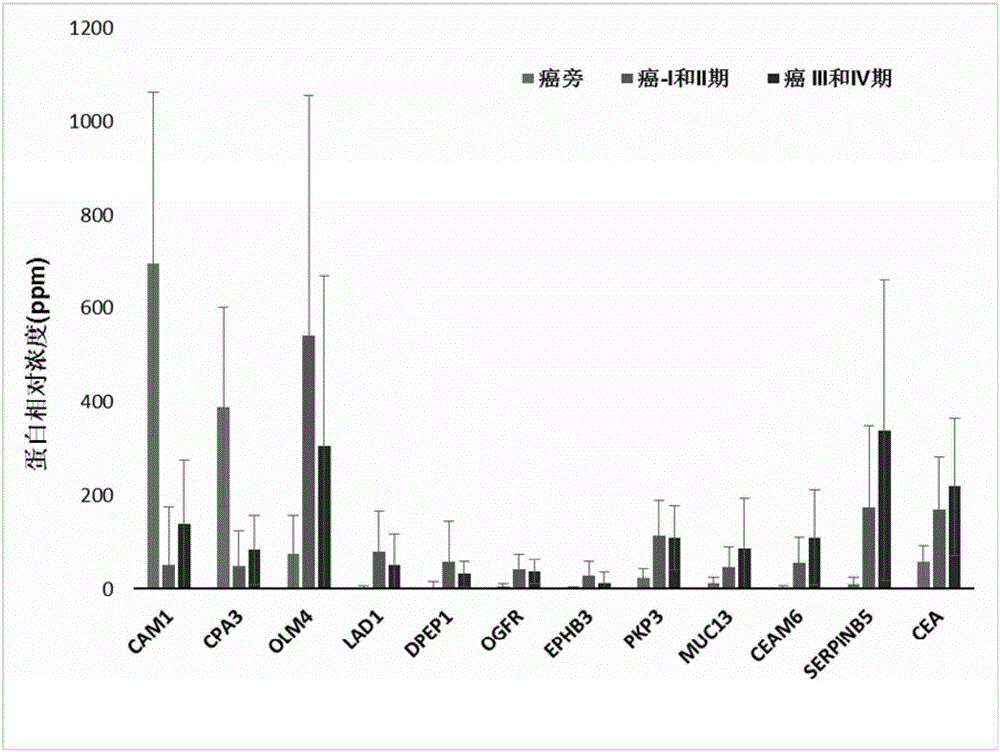
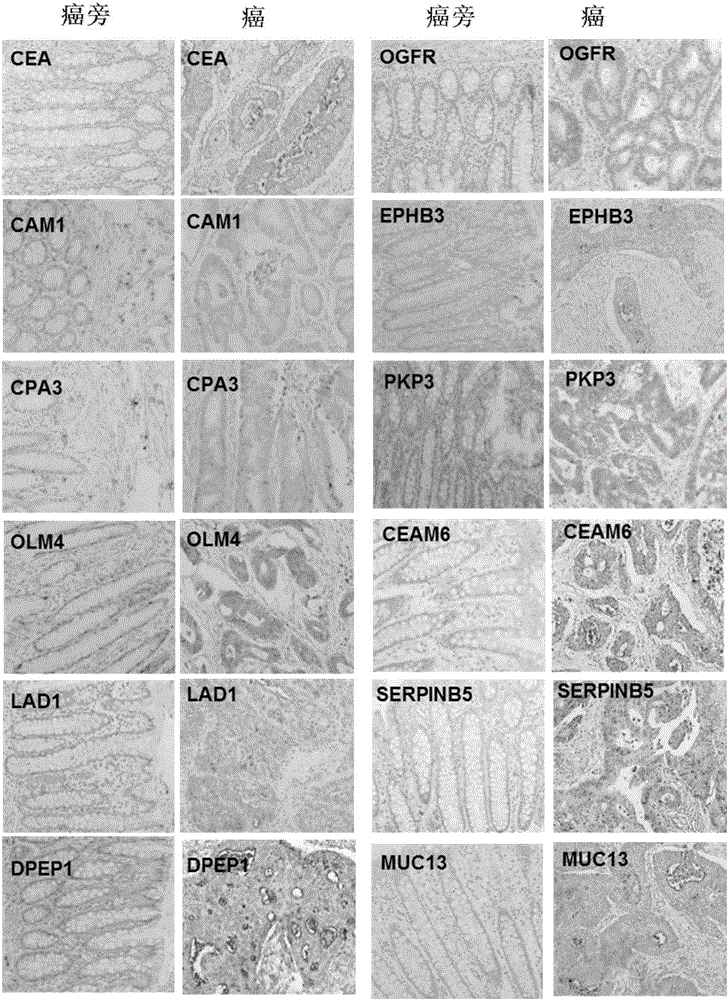
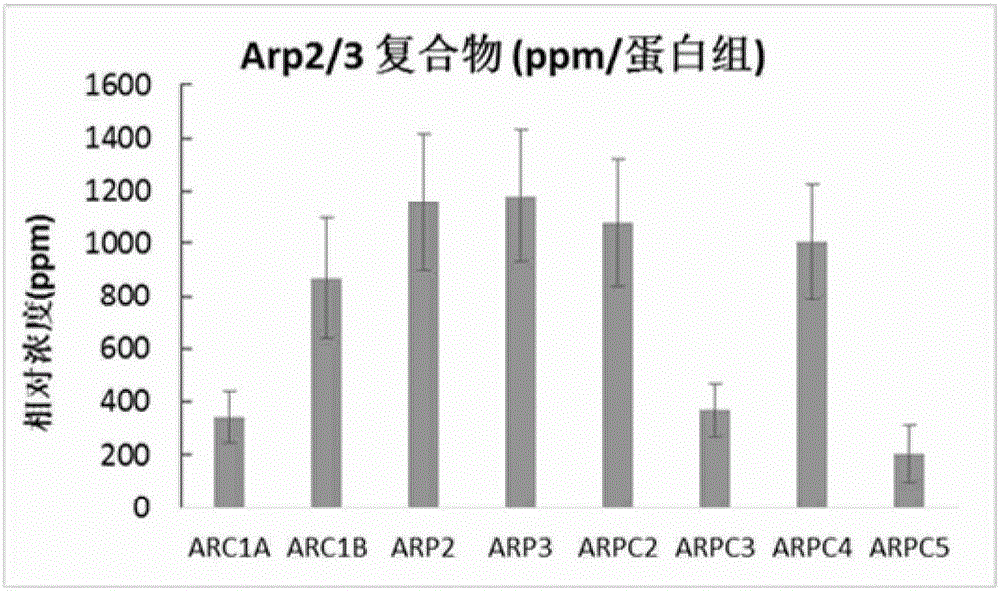
[0095] Example 1: Cancer and paracancerous tissue samples. All samples were collected from clinical patients in accordance with the regulations of the Medical Ethics and Human Clinical Trials Committee of the Affiliated Hospital of Nantong University. After the patient's operation, the cancer and paracancerous tissue samples were placed in different tubes and kept on ice during the subsequent sample transfer process; the samples were finally placed at -80°C for subsequent processing. Paracancerous tissue samples were obtained from at least 5 cm away from the cancerous tissue lesion. A total of 22 pairs of cancer and paracancerous tissue samples were obtained from 22 patients, of which 10 had lymph node metastasis and 12 had no metastasis (Table 1). All patients had been identified as colon or colorectal cancer by pathologists using histological means. Each patient had a detailed pathological record, and those who had various other malignant or infectious diseases, or underwe...
example 2
[0096] Example 2: Protein extraction and separation and in-gel trypsin hydrolysis. Total protein was extracted from fresh frozen tissue using the following method. Cut 0.05 to 0.1 g of frozen tissue into small pieces of 1 mm square with a clean blade, and then transfer to a 1.5 ml centrifuge tube; add 0.4 ml of lysate (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1mM Na2EDTA, 1mM EGTA, 1% Triton X-100, Protease inhibitor cocktail pill); Homogenize with Tang’s homogenizer; add 50ul 10% SDS and 50ul1M DTT to the tube after homogenization, heat at 95°C for 10min; then Sonication is performed to break up the DNA in the sample. The sonicated mixture was centrifuged at 15,000×g for 10 min; the supernatant was transferred to a new tube and stored at -80°C for subsequent analysis. The protein concentration of the supernatant was measured using BCA from Pierce / Thermo Scientific TM Kit OK.
[0097] Gradient gel electrophoresis separation (133ug protein, NuPAGE4-12% Bis-Tris Gel, Life Techno...
example 3
[0098] Example 3: LC / MS / MS analysis. Sixteen small tryptic peptides from one sample were sequentially analyzed by mass spectrometry; the equipment we used was a ThermoScientific Q-Exactive hybrid Quadrupole-Orbitrap mass spectrometer coupled with a Thermo Dionex UltiMate 3000RSLCnano system. The small peptide samples obtained by trypsin hydrolysis were added to a peptide collection tube at a rate of 5ul / min, and the collected peptides were collected at a rate of 0.3ul / min using a 3-36% linear acetonitrile (dissolved in 0.1% formic acid) gradient. Washed into a 25 cm long C18 reverse column (New Obietive, Woburn, MA) over 110 minutes. The small peptides eluted from the reverse column were ionized and sprayed into the mass spectrometer; we used Thermo's Nanospray Flex Ion Source ES071 for ionization under the following conditions: voltage, 1.6kV, temperature, 250°C. The QExactive mass spectrometer operates in a data-dependent mode and can automatically switch between full-scan ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| cover factor | aaaaa | aaaaa |
| cover factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com