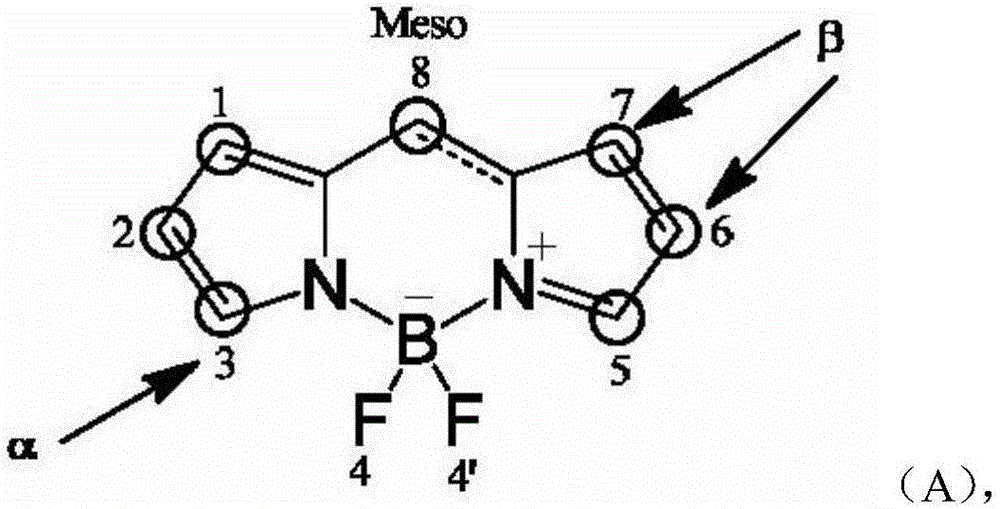
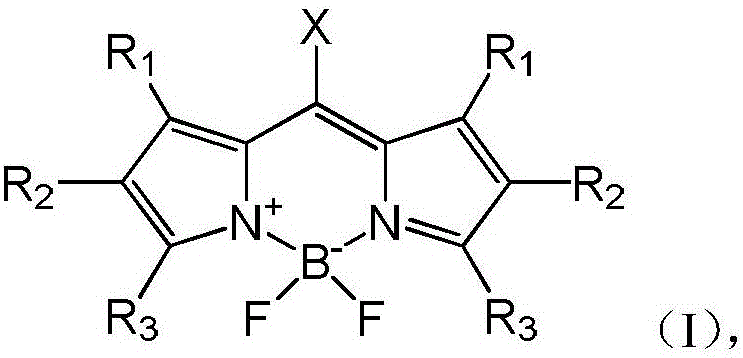
Organosilicon group-modified fluorine-boron dipyrrole fluorochrome and preparation method and application thereof
A fluoroborondipyrrole and a fluoroborondipyrrole technology are applied in the field of organosilicon group-modified fluoroborondipyrrole fluorescent dyes and the preparation thereof, which can solve the problems of no report on the organosilicon group-modified system, and achieve easy industrialization. Production, high molar extinction coefficient, low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
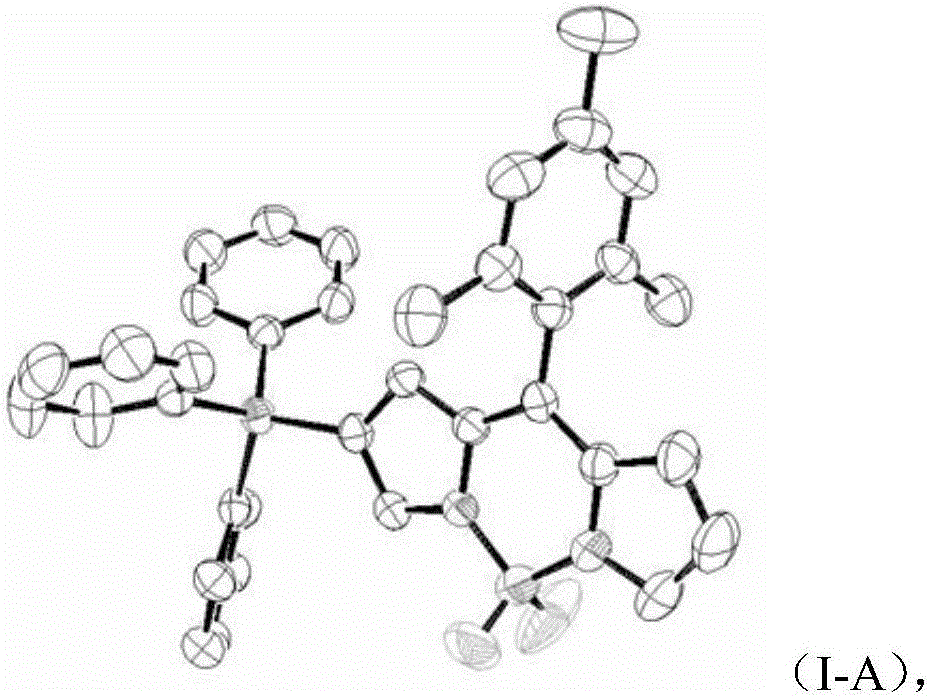
[0022] In a (10ml) schlenk reaction tube, add monoiodoBODIPY (100mg, 0.230mmol), triphenylsilane (299mg, 1.15mmol), ditri-tert-butylphosphine palladium (5.88mg, 0.012mmol) under nitrogen Under protection, 2 ml of toluene and triethylamine (0.037 ml, 0.345 mmol) were added to adjust the alkaline environment, and stirred at room temperature for 1 day. Separation with a chromatographic column (EA:HEX=1:19) can give the product as a bright yellow solid, the single crystal diffraction pattern is shown in (I-A), and the structural formula is as shown in (I-1) Organosilicon group modified fluorine Borodipyrrole Fluorescent Dye 1.
[0023]
[0024] 1H NMR (400MHz, CDCl3) δ7.93(s, 1H), 7.88(s, 1H), 7.49(dd, J=7.9, 1.4Hz, 6H), 7.44-7.40(m, 3H), 7.35(dd, J=11.2, 4.4Hz, 6H), 6.93(s, 3H), 6.70(d, J=4.1Hz, 1H), 6.49(d, J=3.0Hz, 1H), 2.34(s, 3H), 2.12( s, 6H).
[0025] HR-MS: Calcd. For C 36 h 31 BF 2 N 2 NaSi[M+Na] + 591.2216. Found 591.2212
[0026] The synthetic reaction equat...
Embodiment 2
[0029] In one (10ml) schlenk reaction tube, add 2,6-diiodoBODIPY (100mg, 0.178mmol), triphenylsilane (463mg, 1.78mmol), ditri-tert-butylphosphine palladium (9.20mg, 0.0178mmol ), under nitrogen protection, 2 ml of toluene and diisopropylethylamine (0.058 ml, 0.534 mmol) were added to adjust to an alkaline environment, and stirred at room temperature for 2 days. Separation with a chromatographic column (EA:HEX=3:97) can give the product as a bright yellow solid, the single crystal diffraction pattern is shown in (I-B), and the structural formula is shown in (I-2) Organosilicon group modified fluorine Boron dipyrrole fluorescent dye 2.
[0030]
[0031] 1 H NMR (400MHz, CDCl 3 )δ: 7.94(s, 1H), 7.82(s, 1H), 7.49-7.47(m, 6H), 7.45-7.41(m, 3H), 7.38-7.34(m, 6H), 7.01(s, 1H) , 6.93(s, 2H), 6.78(s, 1H), 2.33(s, 3H), 2.11(s, 6H).
[0032] HR-MS: Calcd. For C 54 h 45 BF 2 N 2 NaSi 2 [M+Na] + 849.3084. Found 849.3080
[0033] The synthetic reaction equation is as follows: ...
Embodiment 3
[0036] In a (10ml) schlenk reaction tube, add monoiodo BODIPY (100mg, 0.230mmol), triethylsilane (0.043ml, 0.276mmol), ditri-tert-butylphosphine palladium (7.84mg, 0.016mmol), in Under nitrogen protection, 2 ml of dichloromethane and triethylamine (0.037 ml, 0.345 mmol) were added to adjust the alkaline environment, and stirred at room temperature for 2 days. Separation with a chromatographic column (EA:HEX=1:19) can give organosilicon group-modified fluorobodipyrrole fluorescent dye 3 with the structural formula (I-3).
[0037] The synthetic reaction equation is as follows:
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com