A kind of octasulfonic acid phthalocyanine and its preparation method and application
A technology of octasulfonic acid group and disulfonic acid group, which is applied in the field of octasulfonic acid group-substituted phthalocyanine metal complexes and its preparation, can solve problems such as poor biological selectivity, poor stability, and complicated synthetic routes, and achieve Effects of high photodynamic anticancer activity, large molar absorption coefficient, and high photosensitivity activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] In the present invention, the preparation method of tetra-a-(6,8-sodium disulfonate base-2-naphthyloxy) phthalocyanine metal complex comprises the following steps:
[0037] (1) Preparation of 3-(6,8-disulfonic acid-2-naphthyloxy) phthalonitrile dipotassium salt: 3-nitrophthalonitrile and 2-naphthol-6,8-di Dipotassium sulfonate was used as a reactant, dimethyl sulfoxide was used as a solvent, in the presence of potassium carbonate and under the protection of nitrogen, the reaction was stirred at room temperature ~45°C for 48~96 hours, monitored by thin layer chromatography, when 3-nitro-o When phthalonitrile is basically consumed, the reaction is terminated, and the target product is purified by solvent method, recrystallization method and extraction method; in the above reaction, 3-nitrophthalonitrile and 2-naphthol-6,8-disulfonic acid disulfide The molar ratio of potassium is 1:1 ~ 1.5, the solvent consumption is that every mmol reactant 3-nitrophthalonitrile needs 2 ~...
Embodiment 1
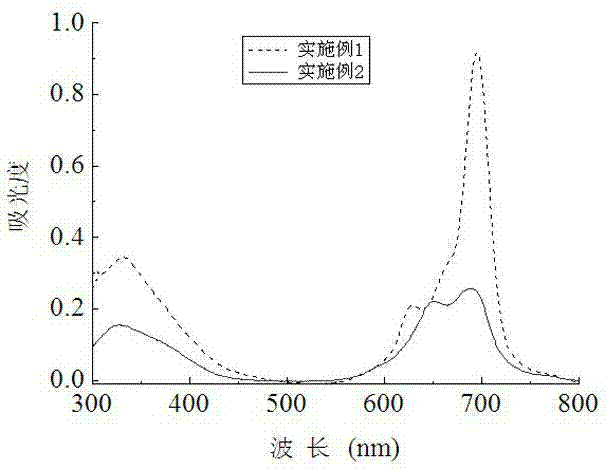
[0051] Synthesis and Physicochemical Properties of 1,8(11), 15(18), 22(25)-tetrakis(6,8-disulfonic acid sodium-2-naphthyloxy)zinc phthalocyanine
[0052]
[0053] Formula 1)
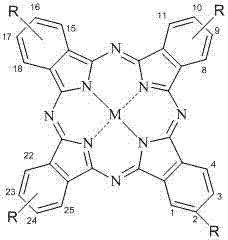
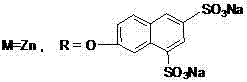
[0054] This compound can also be called tetra-a-(6,8-sodium disulfonate-2-naphthyloxy)zinc phthalocyanine, and its structure is shown in formula (1), wherein:
[0055] .
[0056] (1) Preparation of 3-(6,8-disulfonic acid-2-naphthyloxy) phthalonitrile dipotassium salt: with 3-nitrophthalonitrile (5 mmol) and 2-naphthol-6, Dipotassium 8-disulfonate (5~7.5mmol, preferably 5mmol) was used as reactant, dimethyl sulfoxide (10~25mL, preferably 10mL) was used as solvent, in potassium carbonate (7.5~15mmol, preferably 10mmol), divided into three In the presence of batch addition) and nitrogen protection, the reaction was stirred at room temperature to 60°C (preferably 45°C) for 48 to 96 hours, monitored by thin-layer chromatography, and the reaction was terminated when 3-nitrophthalonitrile was basically c...
Embodiment 2
[0061] Synthesis of 2(3), 9(10), 16(17), 23(24)-tetrakis(6,8-disulfonate sodium-2-naphthyloxy)zinc phthalocyanine
[0062]
[0063] Formula (2)
[0064] This compound can also be called tetrakis-b-(6,8-sodium disulfonate-2-naphthyloxy)zinc phthalocyanine, and its structure is shown in formula (2), wherein:
[0065]
[0066] Substituting equimolar 4-nitrophthalonitrile for 3-nitrophthalonitrile in Example 1, the corresponding peripheral octasulfonic acid group substituted phthalocyanine metal complexes, i.e. four-beta- Zinc (6,8-sodium disulfonate-2-naphthyloxy)phthalocyanine. The structure of the obtained product is the same as that of the phthalocyanine product described in Example 1, except that the position of the substituent is replaced by the β position.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com