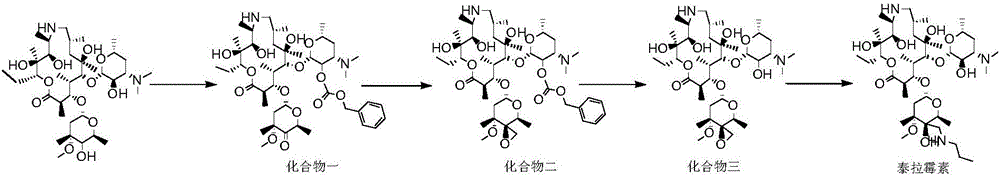
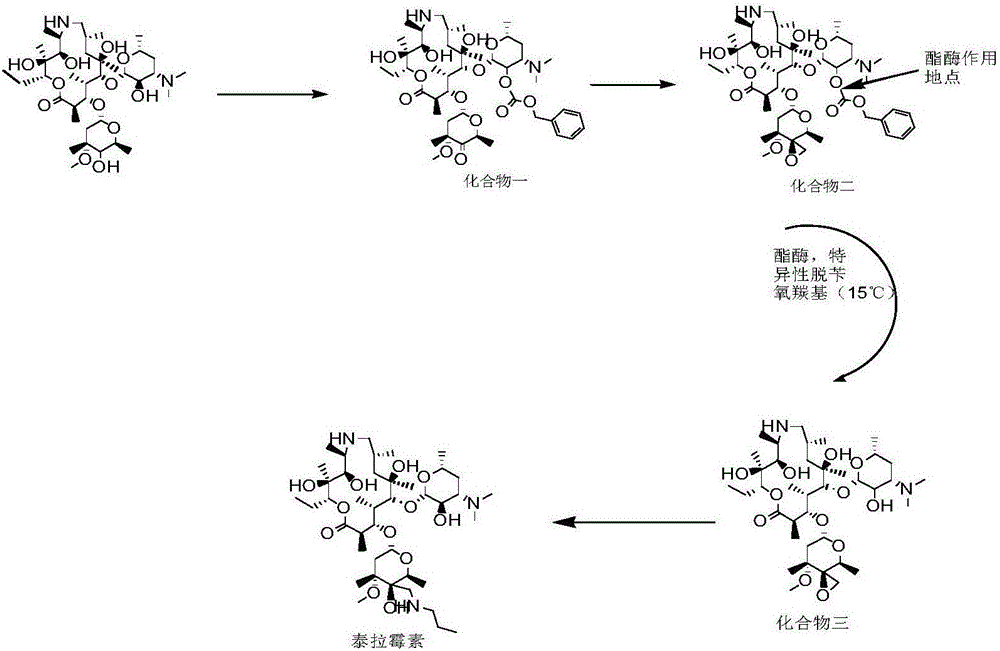
Technology for enzymatic synthesis of tulathromycin
A teramycin and enzymatic synthesis technology, applied in fermentation and other directions, can solve the problems of cumbersome production process, serious pollution, dangerous operation, etc., and achieve the effects of high product purity, less pollution and less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Add 140ml of dichloromethane, 17g (23.1mmol) of demethylated azithromycin, 4.6g (27.6mmol) of benzyl chloroformate into the reaction flask, control the temperature at 0-5°C for 1h, cool down to -80°C, add dimethyl 32g (409.5mol) of sulfoxide, 16.2g (77.0mmol) of trifluoroacetic anhydride was added dropwise, the reaction was completed for 30min, and 17.2g (170.0mmol) of triethylamine was added dropwise for 30min, after the reaction was completed, 100ml of water was added to the system for extraction. The organic phase was concentrated under reduced pressure to obtain 23.8 g of compound one.
[0040] Add 130ml THF, 20.8g (132mmol) trimethylsulfide bromide, 19.6g (175mmol) potassium tert-butoxide to the reaction flask, carry out sulfur ylide reaction at -10~15°C, react for 1.5h and cool down to -80°C, put Compound 2 was dissolved in 50ml of dichloromethane, added to sulfur ylide solution, and reacted at -75 to -85 for 2.5 hours. After the reaction was completed, the reacti...
Embodiment 2
[0043]140ml of dichloromethane, 17g (23.1mmol) of demethyl azithromycin, 4.6g (27.6mmol) of benzyl chloroformate were added to the reaction flask, the temperature was controlled at 0-5°C and reacted for 1h, cooled to -80°C, and dimethyl 32g (409.5mol) of sulfoxide, 16.2g (77.0mmol) of trifluoroacetic anhydride were added dropwise, the reaction was completed dropwise for 30min, 17.2g (170.0mmol) of triethylamine was added dropwise for 30min, and 100ml of water was added to the system for extraction after the reaction was completed. The organic phase was concentrated under reduced pressure to obtain compound-23.5 g.
[0044] Add 130ml of THF, 20.8g (132mmol) of trimethyl sulfide bromide, 19.6g (175mmol) of potassium tert-butoxide into the reaction flask, carry out sulfur ylide reaction at -10~15°C, and cool down to -80°C for 1.5h. The compound was dissolved in 50ml of dichloromethane and added to the sulfur ylide solution, -75~-85 was reacted for 2.5h, after the reaction was com...
Embodiment 3
[0047] 140ml of dichloromethane, 17g (23.1mmol) of demethyl azithromycin, 4.6g (27.6mmol) of benzyl chloroformate were added to the reaction flask, the temperature was controlled at 0-5°C and reacted for 1h, cooled to -80°C, and dimethyl 32g (409.5mol) of sulfoxide, 16.2g (77.0mmol) of trifluoroacetic anhydride were added dropwise, the reaction was completed dropwise for 30min, 17.2g (170.0mmol) of triethylamine was added dropwise for 30min, and 100ml of water was added to the system for extraction after the reaction was completed. After the organic phase was concentrated under reduced pressure, compound one 23.6 was obtained.
[0048] Add 130ml of THF, 20.8g (132mmol) of trimethyl sulfide bromide, 19.6g (175mmol) of potassium tert-butoxide into the reaction flask, carry out sulfur ylide reaction at -10~15°C, and cool down to -80°C for 1.5h. Compound 1 was dissolved in 50ml of dichloromethane and added to the sulfur ylide solution, -75~-85 was reacted for 2.5h, after the react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com