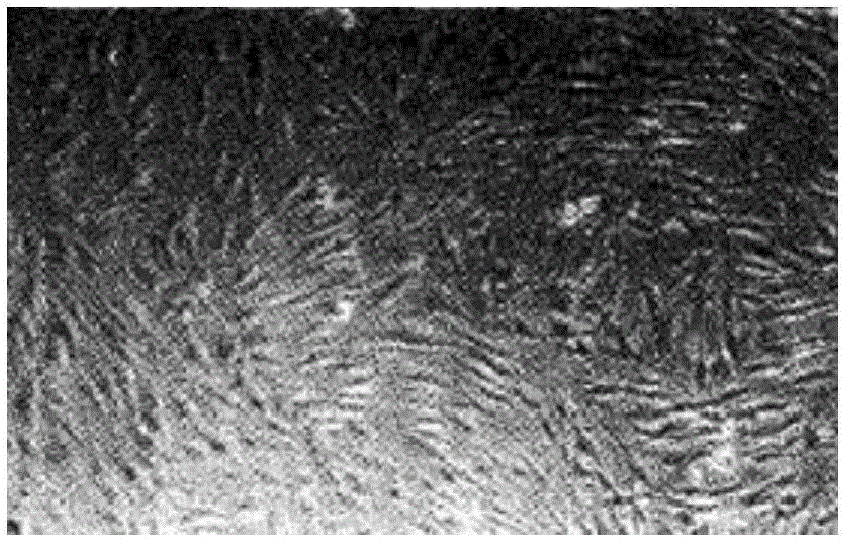
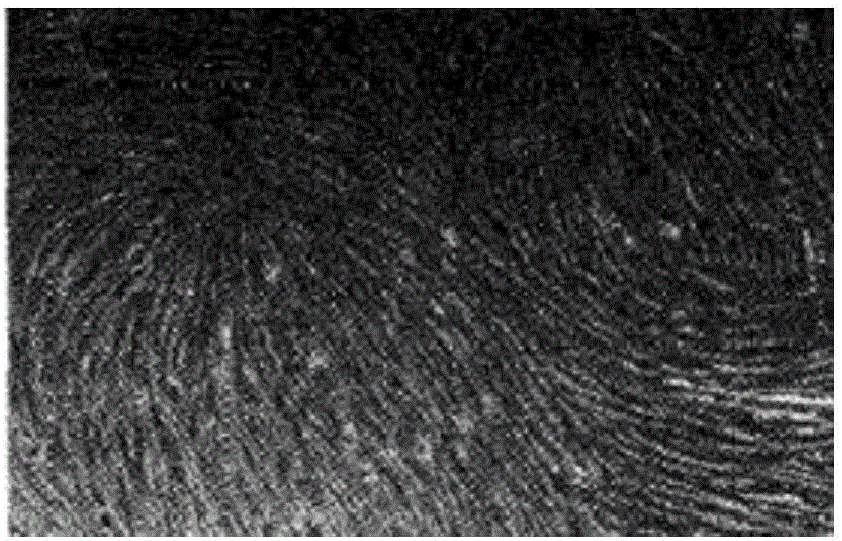
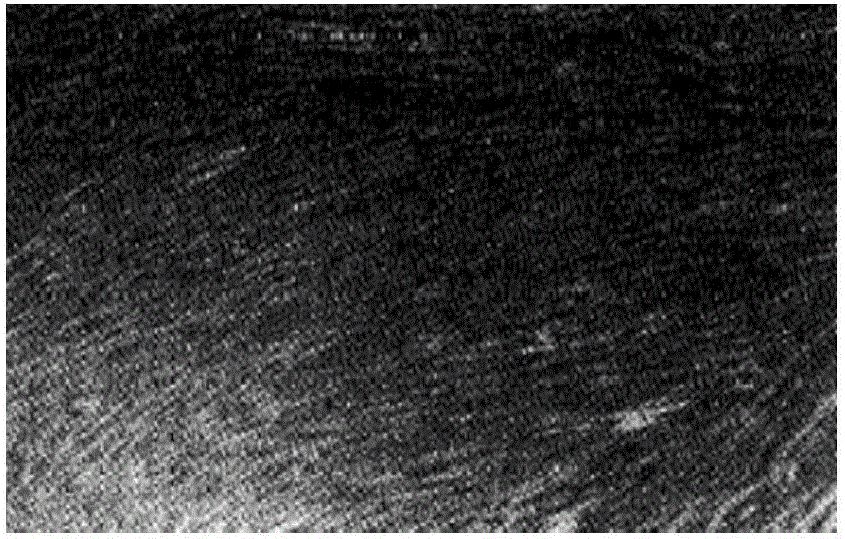
Method for carrying out in-vitro large-scale culture on umbilical cord mesenchymal stem cells
A technology of quality stem cells and umbilical cords, applied in the field of stem cell culture, to achieve the effects of improving adsorption and growth speed, ensuring biogenicity and safety, and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Collect the umbilical cord and cord blood of full-term infants according to national standards, and disinfect the umbilical cord with alcohol;
[0040] (2) Wash off most of the residual blood with fresh buffer solution (containing 1.00 v / v% penicillin / streptomycin), and retain more than 10 v / v% of residual uncoagulated blood. The umbilical cord was crushed using an instrument to obtain 1mm 3 Large and small tissue fragments are digested for a sufficient time in a low-concentration collagenase II environment;
[0041] (3) Discard the remaining tissue pieces after digestion, add eluent and use four-step centrifugation to separate unpurified original umbilical cord mesenchymal stem cells. The parameters of centrifugation are: 300r / min for 10 minutes, 1800r / min for 15 minutes, and 1000r / min10 minutes, 1000r / min10 minutes;
[0042] (4) Count the obtained primary umbilical cord mesenchymal stem cells according to 5.0×10 4 / cm 2 ~1.0×10 5 / cm 2 Planted in a growing ...
Embodiment 2
[0050] Embodiment 2, step (6) in embodiment 1 is pressed 1.0 * 10 7 The concentration per bottle was inoculated in the HYPERFlask culture vessel as compared to 1.25×10 7 / bottle, 1.0×10 7 / bottle, 0.75×10 7 / bottle, 0.5×10 7 The concentration per bottle was inoculated in the HYPERFlask culture vessel.
Embodiment 3
[0051] In Example 3, step (6) in Example 1 was inoculated into a HYPERFlask culture vessel as opposed to being inoculated into a HYPERFlask culture vessel and a common cell culture vessel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com