Method for resolution of R/S-lorcaserin by high performance liquid chromatography method
A technology of high-performance liquid chromatography and chromatographic column, which is applied in the field of high-performance liquid chromatography for the resolution of R/S-lorcaserin, and can solve the problem of poor discrimination between S configuration and R configuration and difficult disassembly of chiral columns Grading problems to achieve the effect of improving peak shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
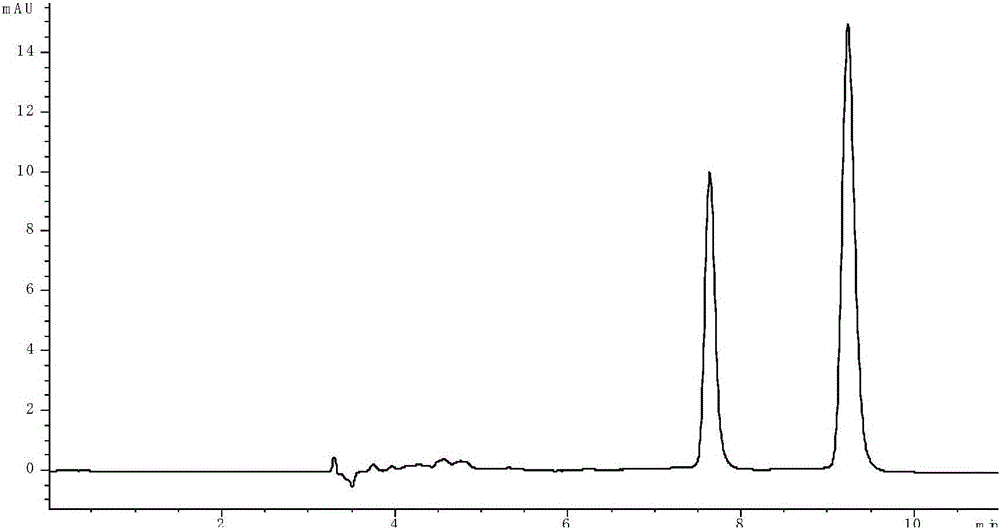
[0052] Embodiment 1: the impact of diethylamine on separation effect
[0053] (1) Take 4mg each of R-lorcaserin and lorcaserin racemate, dissolve them in 1mL methanol solution, dilute 10 times with n-hexane, prepare a solution with a concentration of 400μg / mL, and then take 0.4mL R -Lorcaserin solution and 0.8mL lorcaserin racemate solution are mixed to obtain sample solution, and the content ratio of R-lorcaserin and S-lorcaserin in the solution is 2:1 this moment, then R -The ratio of the chromatographic peak area of lorcaserin and S-lorcaserin should be 2:1;
[0054] (2) An Agilent 1260 high performance liquid chromatograph was used, with an amylose chiral column as the chromatographic column, and n-hexane, ethanol and diethylamine as the normal phase mobile phase. Control the flow rate of the mobile phase to 0.8mL / min, the detection wavelength to 270nm, the temperature of the chromatographic column oven to 35°C, and the injection volume to 10 μL, and adjust the ratio of...
Embodiment 2
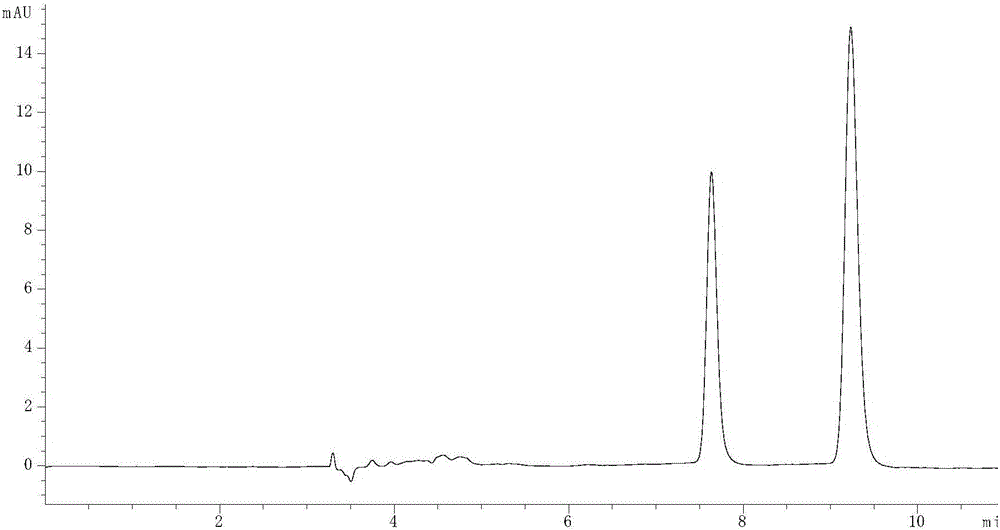
[0062] Embodiment 2: the influence of mobile phase flow rate on separation effect
[0063] (1) Take 4 mg each of R-lorcaserin and lorcaserin racemate, dissolve them in 1 mL of methanol solution, dilute 10 times with n-hexane, prepare a solution with a concentration of 400 μg / mL, and then take 0.4 mL R-lorcaserin solution and 0.8mL lorcaserin racemate solution are mixed to obtain a sample solution, and now the content ratio of R-lorcaserin and S-lorcaserin in the solution is 2:1, then The ratio of the chromatographic peak area of R-lorcaserin and S-lorcaserin should be 2:1;
[0064] (2) Agilent 1260 high performance liquid chromatography is adopted, with amylose chiral column as chromatographic column, n-hexane, ethanol and diethylamine as normal phase mobile phase, calculated by volume percentage, that is, n-hexane: ethanol: Diethylamine is 74.7%:25%:0.3%. The detection wavelength is 270nm, the temperature of the chromatographic column oven is 35°C, the injection volume is...
Embodiment 3
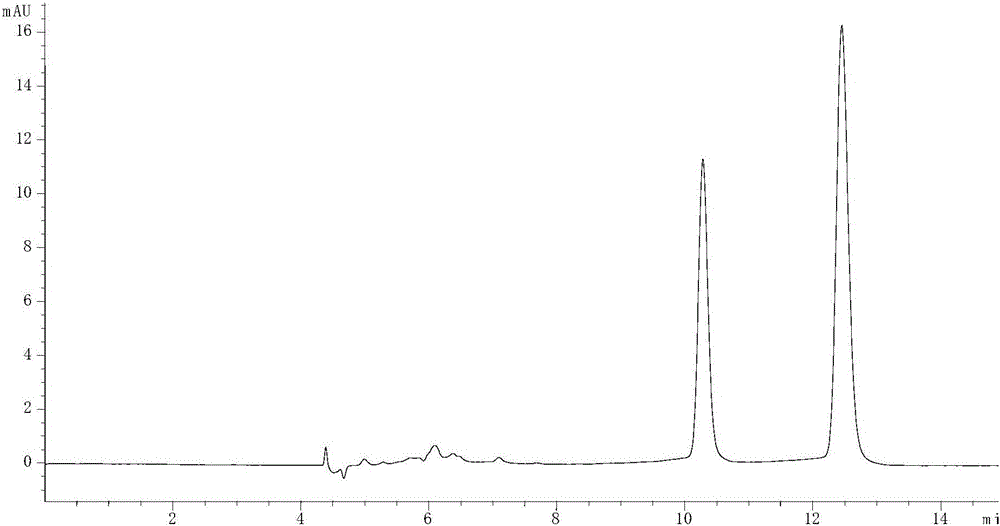
[0072] Example 3: The impact of the type and ratio of alcohol in the normal phase mobile phase on the separation effect
[0073] (1) Take 4mg each of R-lorcaserin and lorcaserin racemate, dissolve them in 1mL methanol solution, dilute 10 times with n-hexane, prepare a solution with a concentration of 400μg / mL, and then take 0.4mL R -Lorcaserin solution and 0.8mL lorcaserin racemate solution are mixed to obtain sample solution, and the content ratio of R-lorcaserin and S-lorcaserin in the solution is 2:1 this moment, then R -The ratio of the chromatographic peak area of lorcaserin and S-lorcaserin should be 2:1;
[0074] (2) adopt Agilent 1260 high-performance liquid chromatograph, take amylose chiral column as chromatographic column, take n-hexane, alcohol and diethylamine as normal phase mobile phase, described alcohol is ethanol or isopropanol; control The flow rate of the mobile phase is 0.8mL / min, the detection wavelength is 270nm, the temperature of the chromatographic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com