Method for separating and determining palbociclib and impurities thereof
An impurity and volume percent technology, applied in the field of analytical chemistry, can solve the problems of difficult to achieve effective separation of Palbociclib and impurities, affecting the quality control of Palbociclib and its preparations, and many impurities in Palbociclib, etc. Achieve the effect of high accuracy, strong specificity, and enhanced retention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
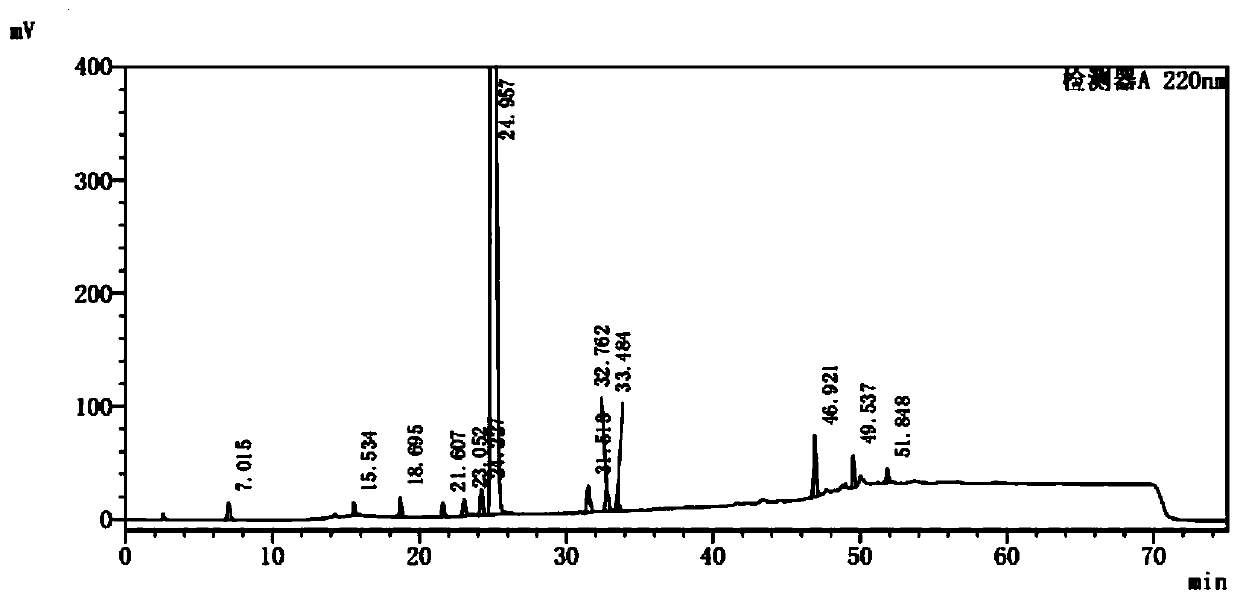
[0040] Take impurity SM1, impurity SM2, impurity A1, impurity Z7, impurity Z8, impurity Z12, impurity Z16, impurity Z21, impurity Z23, impurity Z25, impurity Z26, impurity Z29 (the purity of each impurity is above 95%), each 25mg , accurately weighed, placed in a 25ml measuring bottle, dissolved in methanol-buffer solution (volume ratio 40:60) and diluted to the mark, shaken well, as impurity stock solution; take about 20mg of palbociclib, accurately weighed , placed in a 25ml measuring bottle, accurately add 0.25ml of impurity stock solution, add methanol-buffer solution (volume ratio 40:60) to dissolve and dilute to the mark, shake well, as a mixed control solution.
[0041] Take diluent methanol-buffer solution (volume ratio is 40:60) and mixed control solution respectively, carry out liquid chromatography analysis according to the above-mentioned chromatographic conditions, record the chromatogram, the result is as follows: figure 1 , figure 2 shown.
[0042] figure 1 ...
Embodiment 2
[0044] The determination of embodiment two palbociclib bulk drug (provided by Chongqing Sansheng Industrial Co., Ltd.)
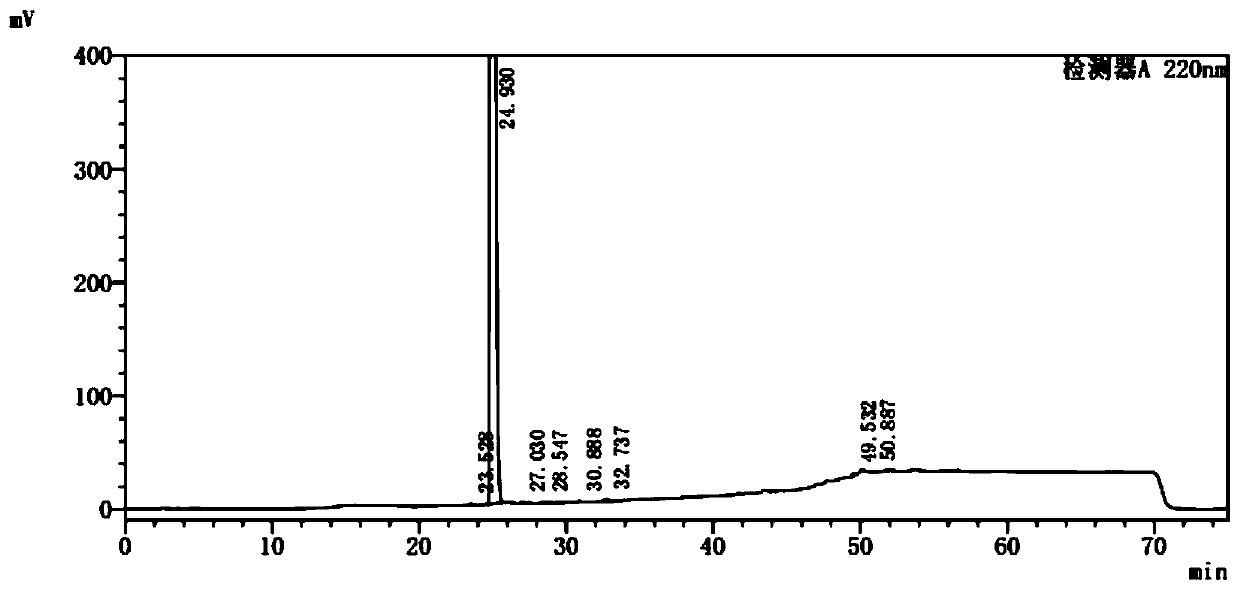
[0045] Take palbociclib 20mg, weigh it accurately, place it in a 25ml measuring bottle, add methanol-buffer solution (volume ratio 40:60) for ultrasonic treatment to dissolve and dilute to the mark, shake well, and use it as the sample solution; Sample solution 1.0ml is placed in 100ml measuring bottle, diluted to scale with methanol-buffer solution (volume ratio is 40:60), shakes up, as contrast solution; Carry out liquid chromatography analysis by the chromatographic condition of embodiment one, record Chromatogram. If there are impurity peaks (except solvent peaks) in the chromatogram of the sample solution, calculate the impurity content according to the self-control method with a correction factor. The result is as image 3 , Figure 4 shown. The test results are shown in Table 1:
[0046] Table 1 palbociclib related substance detection results
...
Embodiment 3
[0049] The determination of embodiment three palbocicini capsules (provided by Chongqing Sansheng Industrial Co., Ltd.)
[0050] Take an appropriate amount of Palbociclib capsules (approximately equivalent to 20 mg Palbociclib), put them in a 25ml measuring bottle, add methanol-buffer solution (40:60 by volume) to dissolve by ultrasonic treatment and dilute to the mark, shake well, filter After that, take the filtrate as the sample solution; accurately measure 1.0ml of the sample solution, place it in a 100ml measuring bottle, dilute it to the mark with methanol-buffer solution (40:60 in volume ratio), shake well, and use it as a control solution; press For the prescription ratio of Palbociclib capsules, take an appropriate amount of blank excipients, and prepare blank excipients for test solution in the same way as the sample solution; carry out liquid chromatography analysis according to the chromatographic conditions of Example 1. Record the chromatogram, the result is as f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com